PowerPoint Presentation
Scene 1 (0s)
[Virtual Presenter] Welcome! Today we are going to be discussing Good Documentation Practices (GDP) and data integrity in the workplace. We will explore how to properly record and store data in accordance with these standards, as well as the importance of adhering to them. Let's get started!.
Scene 2 (21s)
[Audio] It is imperative for healthcare professionals to comprehend and strictly follow Good Documentation Practices (GDPs). GDPs put forward defined regulations to ensure that original data is documented in a clear, trackable, and precise way. The fundamental code of GMP, often referred to as the "Golden Rule of GMP," suggests that if something is not documented, then it did not happen. Appropriate documentation is critical for data precision in the healthcare sector..
Scene 3 (55s)
[Audio] Good documentation practices are essential to ensure reliable and accurate records. It's important to remember that original data must be accurately recorded at the time of observation or measurement in order to prevent bias or potential manipulation of the data. All data must be traceable, accurate, and secure. It is the responsibility of each employee to ensure that data recorded is accurate, complete, and consistent with the work performed. " As part of BioVeritas’ Good Documentation Practices and Data Integrity, each employee is responsible for recording original data at the time of observation or measurement. This is essential to ensure that records are reliable, accurate, and secure. Records must be traceable, precise, and conducted in a consistent manner. By following these guidelines, we can ensure that our data is accurate and secure..
Scene 4 (1m 56s)
[Audio] Good documentation practices are at the core of any research and manufacturing activity, and the scope of this training module provides a framework for best practices related to recording original data in all GMP operations. It is important that the appropriate scope of operations is understood in order to ensure accuracy and legitimacy of the data being recorded. Therefore, it is important to keep in mind that the scope of this training module is limited to activities related to GMP operations, while activities related to Business Development fall outside the scope..
Scene 5 (2m 32s)
[Audio] Adhering to Good Documentation Practices and Data Integrity is critical, as not doing so can lead to Material that cannot be released by Quality Assurance. To prevent this issue from recurring, a root cause investigation should be conducted to identify root causes and contributing factors..
Scene 6 (2m 52s)
[Audio] Original data should be durable and tangible in order to ensure good documentation practices and data integrity. Durability of data is important as it ensures that the data printout is not affected by external elements. Tangibility of data is important as it allows reproducibility of the data in different formats, such as laboratory notebooks, validation protocols, methods qualifications, labels, production records, and computer-generated data. These practices guarantee accuracy and relevance of the recorded data..
Scene 7 (3m 29s)
[Audio] We'll be going over Good Documentation Practices and Data Integrity with respect to recording original data. The table presented shows that original data is often registered in production records, analytical test method forms, commissioning, qualification and validation protocols, labels and laboratory notebooks, as well as glass washer, balance and autoclave printouts. Take into account that Post It notes, scrap paper, laboratory kimwipes and similar articles should not be utilized for the record of original data. In case of any queries, reach out to Quality Assurance for assistance..
Scene 8 (4m 10s)
[Audio] Discussing the importance of good documentation practices and data integrity, we need to look at tools, rules and requirements. For original data, it is preferable to use a blue or black pen. Water-soluble inks and pencils are not allowed. Quality assurance prefers blue pens with a fine point. A Pilot retractable ball point pen with a fine point is compliant, but a Pilot retractable GEL pen is not. Data integrity is essential for consistent, accurate and repeatable results - and good documentation practices are essential to ensure this. We will cover this in more detail in the next slide..
Scene 9 (4m 56s)
[Audio] Poor tool selection for documenting original records can lead to compliance concerns, as is the case with the pen selected for GMP operations in this facility. The pen's cap could cause smearing of legible entries, which would violate regulations. To prevent this, pens with caps that will not cause smearing must be used when recording entries supporting GMP operations. Failure to comply with this requirement will result in a GMP Salute..
Scene 10 (5m 28s)
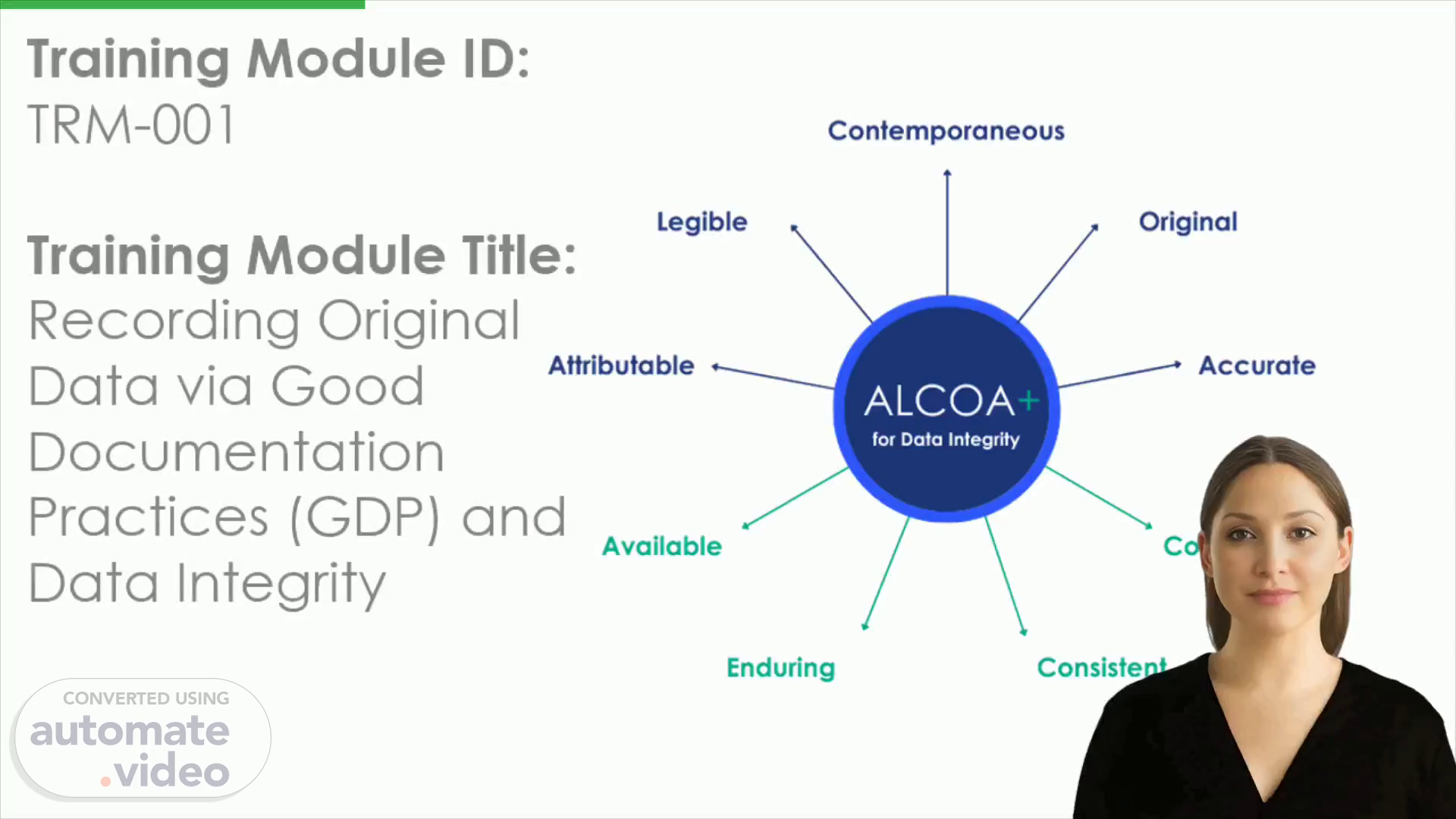
[Audio] Data integrity is an essential part of maintaining reliable records and forensically sound record-keeping processes. Good Documentation Practices, or GDP, provide a framework for recording original data which meets certain criteria including attributability, legibility, contemporaneousness, accuracy, availability, endurance, consistency and completeness. These criteria must be met to ensure the reliable retention of data over the long-term..
Scene 11 (6m 1s)
[Audio] Data integrity and accuracy are of utmost importance, and all records must be attributed to the personnel responsible for the data as well as the date at which it was generated, in compliance with FDA guidelines. Furthermore, data must be traceable or linked back to its source, such as a study, analytical run, or testing system. It is also crucial that the personnel performing the task are properly documented with a corresponding date, to ensure that all documentation complies with FDA regulations and that data integrity is preserved..
Scene 12 (6m 36s)
[Audio] Legible data that is easy to understand is crucial to the accuracy, consistency, and safety of any system. ALCOA+ facilitates complying with this by making the data easier to read. This results in more reliable data, as well as improved record-keeping and reporting. Therefore, good documentation practices and data integrity are essential components of any system..
Scene 13 (7m 5s)
[Audio] Good documentation practices are a must for maintaining data integrity in the workspace. Paying attention to detail is imperative to ensure accuracy. If an entry looks suspicious, like in this case, it's hard to tell if it's a 4 or 9, it's important to verify it and raise any queries. This is essential to keeping up good standards and practices of work. Consistently checking data entries, and asking questions when it looks wrong, is fundamental for making sure accurate data is kept. Keeping to good documentation practices and being mindful of detail is critical for data integrity in the workspace..
Scene 14 (7m 48s)
[Audio] Emphasizing the importance of using proper documentation practices to ensure the accuracy and validity of our data, we must not have a strikethrough for signatures, initials and log legible characters. Furthermore, Quality Assurance will be connecting with GMP personnel to register their initials and to ensure that neither slash is used for zero or seven, as this could appear as an error. Dedicating time to ensuring that the documentation process is done properly will guarantee the integrity of our data and avoid any potential misunderstandings arising from errors..
Scene 15 (8m 25s)
[Audio] Data recorded in the table must be done correctly in order to maintain its integrity. Emphasis is placed on entering the correct value (in this example, 25 mL) as well as entering initials and date for any corrections. The date should display a timestamp of the time the correction was made, and any corrections that are not immediately obvious should be explained in the last column. It is imperative that the accuracy of the data recorded in the table is upheld..
Scene 16 (8m 57s)
[Audio] Proper documentation practices and data integrity are key in guaranteeing that all data, including those related to error correction, is registered accurately. Error correction should always be documented with the initials of the responsible individual, the date and the motive of the correction, if not apparent. The following table displays a few cases of incorrect error correction – changing a 5 to an 8 by writing over it, striking out the error, or not including the initials and date. All of the aforementioned errors can lead to inaccurate data. This is why it is essential to ensure proper error correction from the start..
Scene 17 (9m 39s)
[Audio] Errors that happen when original data is recorded must be fixed as part of Good Documentation Practices (GDP) and Data Integrity. Even though there is no expected perfection when making those records, errors should still be corrected as the GDP requires. When an entry made is connected to GMP Operations, it is important to use GDP and ensure data integrity by making the necessary corrections..
Scene 18 (10m 8s)
[Audio] Documentation practice is essential when recording original data. It is important to comprehend when and how to make alterations. As seen in the example, the date 4/11/23 is documented as it is an amendment to the already noted date 5/11/23. Additionally, protocol necessitates that all changes must be indicated with initials and the date. Following these steps allows us to guarantee the precision of the registered data..
Scene 19 (10m 41s)
[Audio] Data reliability is key for proper documentation and data integrity. Recording dates accurately can be difficult, and the format will depend on the software. It is important to remember that the format variations given below are acceptable. For instance, April 11, 2023 can be recorded as 4/11/23, 04/11/2023, 4-11-23 or 04-11-2023. Using the correct formats can help guarantee the accuracy of initial data recording..
Scene 20 (11m 19s)
[Audio] Acceptable time formats for recording accurate data are both 12 hour and 24 hour formats. As an example of 12 hour format, 3:05 p.m. can be noted as 3:05 p.m. or 1505 hours. A revised policy regarding time formats will be provided soon, so it is important to become familiar with these formats now..
Scene 21 (11m 48s)
[Audio] As professionals, it is our responsibility to use good documentation practices (GDP) and adhere to data integrity principles. This means that any entries made into our systems must be documented, accurate, and authenticated. This table outlines the important components to review when making corrections. Initials, date, and reason for correction are essential for proper tracking and auditability. Additionally, the original recorded value must be visible to the reviewer, and as specified, these items are not acceptable. Let us ensure accurate documentation and data integrity for our customers..
Scene 22 (12m 32s)
[Audio] As stated in this slide, ALCOA+ Contemporaneous must be accurately recorded at the time the work is performed, and Compliance JDO must be performed and the value recorded on April 11, 2023. This is essential to ensure Good Documentation Practices and Data Integrity. Strict adherence to these protocols will ensure accuracy in data processing and reporting..
Scene 23 (12m 58s)
[Audio] In order to maintain data integrity and comply with Good Documentation Practices, it is essential that all records connected to pre-filling processes be accurately documented. To safely verify accuracy, all pre-filling activities must be documented as they are performed. Additionally, pre-filling any information should be avoided, as it could lead to data integrity risks..
Scene 24 (13m 25s)
[Audio] Recording original data via Good Documentation Practices (GDP) and Data Integrity is an important step. Pre-filling and post-filling data and information in a way that accurately describes what happened and when is imperative. When post-filling, clear explanations should be given for why the information was not recorded promptly and these post-filling entries should be kept to a minimum..
Scene 25 (13m 51s)
[Audio] GDP emphasizes the importance of proper documentation when recording original data. This is essential for maintaining data integrity. If insufficient space exists next to an error then it must be corrected at a suitable location. In this case, a single line should be drawn across the error and a reference number circled next to it. At the new location, the same reference number, correction, initials, date, and explanation of the correction must all be documented. It is important to note that the reference number should reset with each new page. Practicing this process will ensure that it becomes a regular part of your data logging..
Scene 26 (14m 38s)
[Audio] An example of Good Documentation Practices, or GDP, is shown in this slide. It can be seen that the initial entry was incorrect, and a slash has been used to indicate this. Additionally, a reference number is included along with additional comments to give more detail on the value. As this module is being worked through, the use of the reference number should be noted, as well as when a slash is needed to mark an error..
Scene 27 (15m 7s)
[Audio] GDPs, short for Good Documentation Practices, are essential for recording initial data with precision and integrity. This slide explains how to carry out GDPs using the available space on the page or the remarks section of the production record. As an instance, if the page is not enough for all the data, putting the additional details in the remarks section of the production record is an appropriate way. With the implementation of these practices, one can ensure the exactness and reliability of the data..
Scene 28 (15m 42s)
[Audio] Proper documentation procedures are necessary for preserving data accurateness. When transferring information from one source to a Quality Assurance (QA) controlled document, it is important to enter the data immediately into the QA controlled document and dispose of any temporary notes such as post it notes. Doing this gets rid of any risk of writing an incorrect value and also prevents any major compliance matters..
Scene 29 (16m 10s)
[Audio] Gaining accuracy in data collection requires Good Documentation Practices and Data Integrity. This means obtaining the original data sets that are complete and represent the observation in a truthful manner. Additionally, having a second person verify process steps that are deemed most critical to operations will help ensure the entries are accurate. Even if the measurements are found to be out of range, they should be accepted as accurate if they reflect the process. It is important for all staff to understand the importance of GDP and data integrity in order to generate reliable results within the operations..
Scene 30 (16m 52s)
[Audio] Ensuring accuracy and data integrity within the Good Documentation Practices (GDP) is paramount. If any section, field, or table is left blank, it must be closed out with initials, date, and the reason why the data or information was not required. This will help maintain the accuracy and integrity of the data..
Scene 31 (17m 15s)
[Audio] Ensuring that original data is accurately recorded is essential. When entering data, do not use ditto marks in place of completely entering an entry. Even if there is no change in data, ditto marks should not be used, as it has the potential to be misinterpreted and does not guarantee data integrity..
Scene 32 (17m 36s)
[Audio] Documentation practices and data integrity are essential for any organization's operations and should be fully complied with. To ensure accurate and reliable records are created, it is advised to only fill in the fields that are “prescribed”. This enables a consistent, traceable and documented data entry, ultimately benefiting the organization in the long run. Additionally, it guarantees that all pertinent information is accurately recorded and distributed promptly, resulting in a more organized and effective working environment..
Scene 33 (18m 13s)
[Audio] It is essential to practice good documentation to keep data intact. The document should be kept neat and can be read clearly, without any extra marks, sketches, writings, ink smudges, folding, or creasing. To prevent duplication, ditto marks should not be utilized. As a way to be confident of the data, the records should be kept as initially released..
Scene 34 (18m 41s)
[Audio] Data integrity is of utmost importance when recording accurate and relevant data. To ensure accuracy in data recording, it is important to understand the meaning and application of various abbreviations that may be encountered. This slide provides a table of abbreviations, such as N/A meaning Not Applicable, N/R meaning Not Required, S/N meaning Serial Number, and N/S meaning Not Specified, to help ensure that data is accurately recorded. Understanding and correctly applying these abbreviations is essential for accurate data capture..
Scene 35 (19m 19s)
[Audio] Organizations need to maintain good documentation practices to guarantee the accuracy and reliability of data. In this training session, we will analyze how to register primary data in a methodical, chronological order with time stamps. This practice allows the reviewer to understand the flow of the process because of the chronological order it was processed, in particular if it took several days to finish the process. Furthermore, the production document should be structured to run the process with data and information logged in a temporal order of events. Let us look more closely how to reach this..
Scene 36 (20m 0s)
[Audio] Good documentation practices (GDP) are vital for any industry to ensure data integrity and long-term data preservation. Effective implementation of GDP will guarantee accuracy and completeness in data recording, safeguarding it from corruption, destruction, or misinterpretation. Choosing appropriate systems and tools is paramount to guarantee enduring, accurate, and complete documentation of data, in order to protect data integrity and enable better decision-making processes. Accurate and complete documentation is essential for any industry to ensure original data is securely recorded. To achieve this, proper implementation of good documentation practices is necessary to ensure data is accurately and completely recorded, protecting against corruption, destruction, and misinterpretation. Care must be taken when storing data, as systems and tools that guarantee enduring and reliable records are necessary to guarantee data integrity and facilitate more effective decision-making. As an example, pencil entries should be avoided since they can be erased, while tickets printed on thermal papers may be vulnerable to fading due to heat and other harsh environmental elements. By correctly documenting data, it will ensure that no information is lost and the original data is recorded safely..
Scene 37 (21m 29s)
[Audio] It is important to record original data for review and audit purposes as part of Good Documentation Practices and Data Integrity. If required, this data can be retrieved for review. To retain a copy of the original documentation, scanned copies of the document are allowed in GMP audits. All changes made to the original document must be reflected in the Document Revision History. Thank you for taking the time to review this important training module..