Scene 1 (0s)
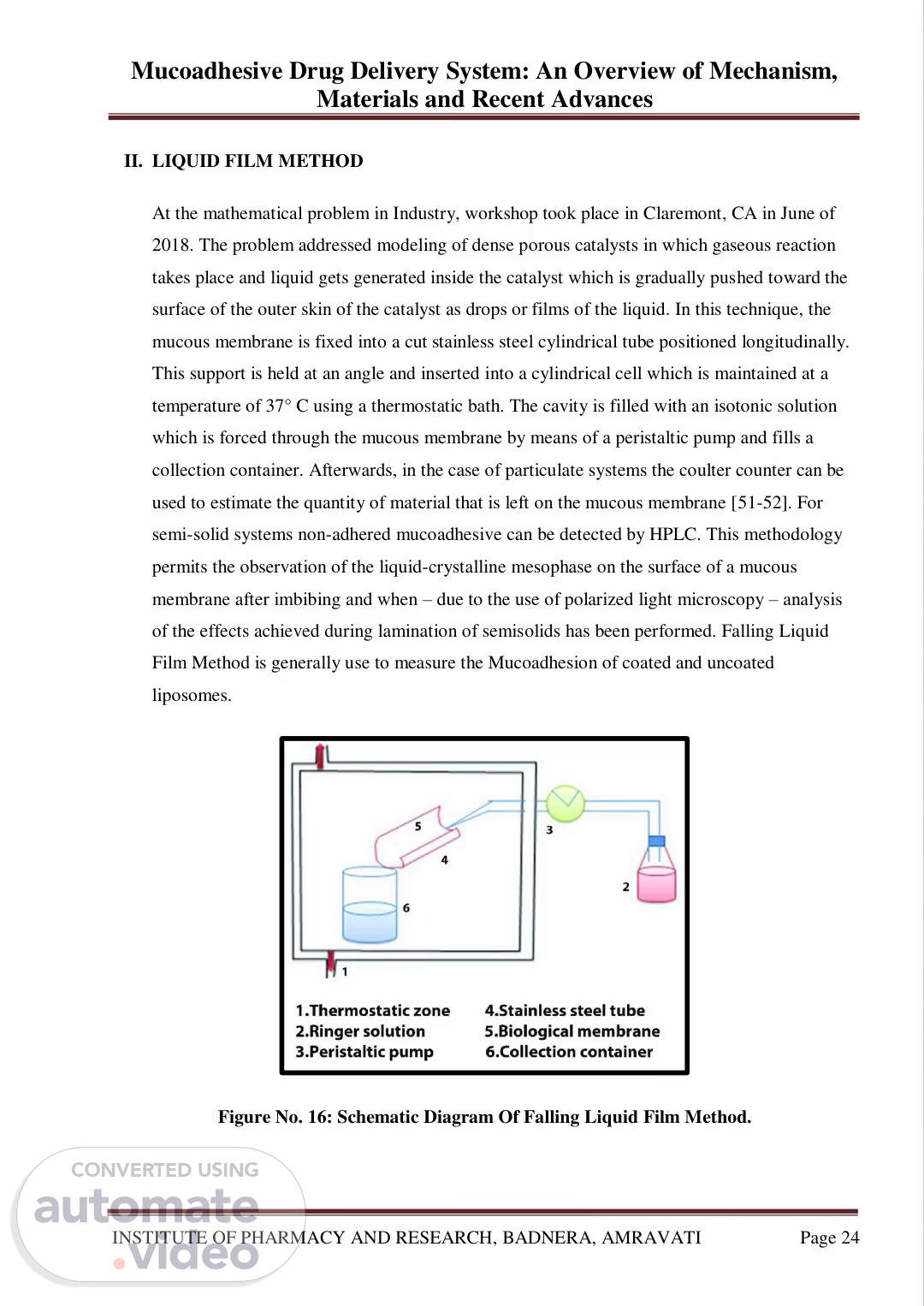
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 24 II. LIQUID FILM METHOD At the mathematical problem in Industry, workshop took place in Claremont, CA in June of 2018. The problem addressed modeling of dense porous catalysts in which gaseous reaction takes place and liquid gets generated inside the catalyst which is gradually pushed toward the surface of the outer skin of the catalyst as drops or films of the liquid. In this technique, the mucous membrane is fixed into a cut stainless steel cylindrical tube positioned longitudinally. This support is held at an angle and inserted into a cylindrical cell which is maintained at a temperature of 37° C using a thermostatic bath. The cavity is filled with an isotonic solution which is forced through the mucous membrane by means of a peristaltic pump and fills a collection container. Afterwards, in the case of particulate systems the coulter counter can be used to estimate the quantity of material that is left on the mucous membrane [51-52]. For semi-solid systems non-adhered mucoadhesive can be detected by HPLC. This methodology permits the observation of the liquid-crystalline mesophase on the surface of a mucous membrane after imbibing and when – due to the use of polarized light microscopy – analysis of the effects achieved during lamination of semisolids has been performed. Falling Liquid Film Method is generally use to measure the Mucoadhesion of coated and uncoated liposomes. Figure No. 16: Schematic Diagram Of Falling Liquid Film Method..
Scene 2 (1m 0s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 25 III. FLUORESCENT PROBE METHOD Pyrene and fluorescein isothiocyanate are utilized in this method as markers for the lipid bilayer membrane and membrane proteins respectively. The mucoadhesive agents are combined with cells, followed by observing changes in the fluorescence spectra. This indicates polymer binding and its function in polymer adhesive properties. Figure No. 17: Fluorescent Probe Equipment. IV. MUCIN CONJUGATE METHOD In order to investigate bioadhesion, a colloidal gold staining technique was employed. This technique employs red colloidal gold particles which get adsorbed on mucin molecules to produce mucin-gold conjugates. These conjugates upon interaction with bio adhesive hydrogels acquire reddish color. This can be predicted by either estimating the surface color of the hydrogel as percentage of saturation of the red color or by gauging percentage reduction in concentration of the conjugates by measuring absorbance at 525nm. Swelling index. The degree of swelling is expressed in % increase in weight of the formulation..
Scene 3 (1m 44s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 26 Figure No. 18: Diagram of Colloidal Gold Staining Technique. V] SWELLING INDEX The percentage increase in weight gained by the formulation is used to quantify swelling. It is determined by the formula, Swelling index (S.I.) = (Wt-Wo/Wo) Where, S.I- Swelling index Wt= Weight of tablet at time Wo =Weight of the tablet before placing in beaker. B] ADHESIVENESS MEASURING METHOD It is employed for performing qualitative analysis in peel adhesive strength on the polymer substrate, and easy to apply remedy for developing buccal patch delivery systems. Adhesiveness is quantitated by the strain needed to remove one´s thumb from adhesive when varying pressure and time of contact..
Scene 4 (2m 20s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 27 Figure No. 19: Adhesion Test. C] ELECTRICAL CONDUCTANCE They investigated various semisolid mucoadhesive ointments and modulated the rotational viscometer to predict electrical conductance, which was observed low in the presence of a slightly adhesive due to mineral oil coat. D] STABILITY STUDIES Stability studies: The stability of successful formulation can be established only by these These are done to develop a stable product ensuring safety and efficacy throughout the end of shelf life under defined storage conditions, peak profile being retained. This follows ICH Guidelines. E] RESIDENCE TIME MEASUREMENT / IN VIVO TECHNIQUES Quantitative assessment of the mucoadhesion properties is done by measuring the residence time of a Muco adhesives at an application site [50]. Gl transit times for various Mucoadhesive formulations was carried out using radioisotopes and fluorescent labelling techniques..
Scene 5 (3m 0s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 28 i] RADIO-OPAQUE TABLETS IN TRANSIT It is a basic technique in which radio-opaque markers (e.g. barium sulfate) are micro- encapsulated within mucoadhesive tablets, for evaluating the effect of mucoadhesives on GI transit time. ii] GAMMA SCINTIGRAPHY TECHNIQUE Technetium-labeled tablets (HYAFF): In this study the radioactivity in genital tract was evaluated after hydrating of HYAFF-based biomaterial. Retention of the mucoadhesive- radio labeled tablets with HYAFF based polymer was higher in dry form than [53]. 3. RECENT ADVANCES IN MUCOADHESIVE DRUG DELIVERY SYSTEM I] MUCOADHESIVE BUCCAL TABLETS: These are similar to conventional tablets, but they have the property of mucoadhesion and instead of swallowing, they held in between cheeks and gums. These tablets are sufficiently dissolved by the medium, provided from locations where they are placed, the dissolution of tablet should be slowing order to ensure a sustained and controlled release. Buccal cavity and gets softened due to the production of saliva continuously in the mouth and which ensures the complete drug release in the systemic circulation through the blood capillaries in the buccal cavity and thereby by-passes the hepatic metabolism. Eg. Testosteron, Zolpidem, Fentanyl Citrate [54]. Figure No. 20: Administration of Buccal Tablet..
Scene 6 (3m 55s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 29 II] BUCCAL CHEWING GUM: Medicated chewing gum is particularly used in the treatment of oral cavity and in nicotine replacement therapy. Buccal patches are preferred as best mucoadhesive buccal drug delivery system because of its flexibility and patient comfort. Eg: - Nefidipine, Nimesulide, ketoprofen, baclofen [55]. Figure No. 21: Medicated Chewing Gum. III] GELS: Gels have been studied as a method of controlled medication delivery. Bioadhesive controlled drug delivery's main objective is to localise a delivery device inside the body to improve medication absorption in a targeted way. The characteristics of the polymeric controlled release device, and the presence of the medication itself all interact synergistically to effect bioadhesion. Over half of the medicinal substances and delivery systems now being developed are in the research and development stage, However, the use of gels on the oral mucosa has been debated for the topical administration of antifungal, anti-inflammatory, and mucoprotective medications to the oral mucosa, as well as the systemic administration of analgesics, antihypertensives, and medications for treating cardiovascular disease. Eg: - Progesterone, Carbomer [56]..
Scene 7 (4m 45s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 30 Figure No. 22: Vaginal Formulation in Mucoadhesive Gel. IV] MUCOADHESIVE BUCCAL FILMS: These are mainly referred to transparent drug loaded films which are intended to be placed in the buccal mucosa because of its adhesive character. Buccal films can be more preferred over other dosage forms because of its flexibility and comfortness. The mucoadhesive buccal patches can be of two types: A) MATRIX TYPE: Drug, adhesive and additives mixed together and this mixture is then designed in the form of patches. B) RESERVOIR TYPE: Drug and additives should be separated from the additives. Depending on the presence or absence of a backing membrane, the release from the patch is unidirectional or bi-directional [57]..
Scene 8 (5m 21s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 31 Figure No. 23: Mucoadhesive Buccal Film. V] PATCHES: Drug delivery systems that attach to the oral mucosa and come in a variety of forms have been created. Patches that deliver medications to the mouth cavity through a dissolvable matrix. When used to treat oral candidiasis and mucositis, these patches have a longer duration of action than solid dosage forms like tablets and lozenges. The patches provide the oral mucosa with a regulated, concentrated dosage of the medication for 10–15 hours. During use, they slowly and completely disappear, leaving nothing to be removed. Eg- Insulin, tetracaine, protirelin, tetracycline [58]..
Scene 9 (5m 54s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 32 Figure No. 24: Mucoadhesive Buccal Patches. VI] DEVICES: Several laminated devices have been developed to achieve sustained drug release. It can be classified as: 1. MONOLITHIC (OR MATRIX) SYSTEMS: Where the drug is dissolved or dispersed in the polymer system – diffusion of drug from the drug/polymer matrix controls the overall rate of its release from the device. 2. RESERVOIR (OR MEMBRANE) SYSTEMS: Where diffusional resistance across a polymeric membrane controls the overall drug release rate [59]..
Scene 10 (6m 23s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 33 4. CONCLUSION Mucoadhesive Drug delivery systems designed with the aim to improve patient compliance and convenience is more important than ever. Oral mucosal delivery offers a convenient way of dosing medication, not only to special populations with swallowing difficulties but also to the general population. Mucoadhesive dosage forms provide prolonged contact time at the site of attachment, having high patient compliance and are economic as compared to other dosage forms. Bio-adhesive drug delivery system looks a promising approach to achieve a targeted and sustained release of drug while maintaining patient compliance. mucoadhesive drug delivery system is a good alternative to conventional drug delivery because of its ability to prevent first pass metabolism, enhance bioavailability, and reduce dose frequency. Development of oral mucoadhesive was increased because of their various therapeutic usages. By the introduction of many drug molecules, oral mucoadhesive drug delivery will play a significant role in the delivery of these molecules..
Scene 11 (7m 6s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 34 5. REFERENCES 1. Chickering, D.E. and Mathiowitz, E., 1999. Definitions, mechanisms, and theories of bioadhesion. Drugs and the pharmaceutical sciences, 98, pp.1-10 2. Ahuja, A., Khar, R.K. and Ali, J., 1997. Mucoadhesive drug delivery systems. Drug Development and industrial pharmacy, 23(5), pp.489-515. 3. Woodley, J., 2001. Bioadhesion: new possibilities for drug administration?. Clinical pharmacokinetics, 40, pp.77-84. 4. HäGerström, H., Edsman, K. and Strømme, M., 2003. Low-frequency dielectric spectroscopy as a tool for studying the compatibility between pharmaceutical gels and mucous tissue. Journal of pharmaceutical sciences, 92(9), pp.1869-1881. 5. Andrews, G.P. and Jones, D.S., 2006. Rheological characterization of bioadhesive binary polymeric systems designed as platforms for drug delivery implants. Biomacromolecules, 7(3), pp.899-906. 6. Evangelista, R.C., 2006. Sistemas de liberação controlada de fármacos. Araraquara: Tese. 7. Henriksen, I., Green, K.L., Smart, J.D., Smistad, G. and Karlsen, J., 1996. Bioadhesion of hydrated chitosans: an in vitro and in vivo study. International journal of pharmaceutics, 145(1- 2), pp.231-240. 8. Boddupalli, B.M., Mohammed, Z.N., Nath, R.A. and Banji, D., 2010. Mucoadhesive drug delivery system: An overview. Journal of advanced pharmaceutical technology & research, 1(4), pp.381-387. 9. Trivedi, U.M., Patel, V.M., Mahajan, A. and Mitesh, P., 2011. A review on mucoadhesion, mucoadhesive polymer and mucoadhesive site. Int. J. Institutional Pharm. Life Sci, 1(2), pp.1-18..
Scene 12 (8m 11s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 35 10. Alexander, A., Sharma, S. and Khan, M.J., 2011. THEORIES AND FACTORS AFFECTING MUCOADHESIVE DRUG DELIVERY SYSTEMS: A REVIEW. International Journal of Research in Ayurveda & Pharmacy, 2(4). 11. Patel, R.S. and Poddar, S.S., 2009. Development and characterization of mucoadhesive buccal patches of salbutamol sulphate. Current drug delivery, 6(1), pp.140-144. 12. Ponchel, G., Touchard, F., Duchêne, D. and Peppas, N.A., 1987. Bioadhesive analysis of controlled-release systems. I. Fracture and interpenetration analysis in poly (acrylic acid)- containing systems. Journal of Controlled Release, 5(2), pp.129-141. 13. Samanthula, K.S., Satla, S.R. and Bairi, A.G., 2019. Development, In-Vitro and Ex-Vivo Evaluation of Muco-adhesive Buccal patches of Candesartan cilexetil. Research Journal of Pharmacy and Technology, 12(6), pp.3038-3044. 14. Andrews, G.P. and Jones, D.S., 2006. Rheological characterization of bioadhesive binary polymeric systems designed as platforms for drug delivery implants. Biomacromolecules, 7(3), pp.899-906. 15. Smart, J.D., 2005. The basics and underlying mechanisms of mucoadhesion. Advanced drug delivery reviews, 57(11), pp.1556-1568. 16. Dodou, D., Breedveld, P. and Wieringa, P.A., 2005. Mucoadhesives in the gastrointestinal tract: revisiting the literature for novel applications. European journal of pharmaceutics and biopharmaceutics, 60(1), pp.1-16. 17. Pal, R., Pandey, P., Thakur, S., Chanana, A. and Singh, R.P., 2022. BIODEGRADABLE POLYMER’S ENHANCING DRUG DELIVERY ACTIVITY IN DIFFERENT NOVEL DRUG DELIVERY SYSTEM. WJPPS, 12(1), pp.2046-2069 18. Patel, A.R., Patel, D.A. and Chaudhry, S.V., 2011. Mucoadhesive buccal drug delivery system. International Journal of Pharmacy & Life Sciences, 2(6)..
Scene 13 (9m 16s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 36 19. Okutan, N., Terzi, P. and Altay, F., 2014. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocolloids, 39, pp.19-26. 20. Lee, J.W., Park, J.H. and Robinson, J.R., 2000. Bioadhesive‐ based dosage forms: The next generation. Journal of pharmaceutical sciences, 89(7), pp.850-866. 21. Tiwary, A.K. and Rana, V., 2010. Cross-linked chitosan films: effect of cross-linking density on swelling parameters. Pak J Pharm Sci, 23(4), pp.443-448. 22. Leung, S.H.S. and Robinson, J.R., 1990. Polymer structure features contributing to mucoadhesion. II. Journal of controlled release, 12(3), pp.187-194. 23. Yadav, V.K., Gupta, A.B., Kumar, R., Yadav, J.S. and Kumar, B., 2010. Mucoadhesive polymers: means of improving the mucoadhesive properties of drug delivery system. J. Chem. Pharm. Res, 2(5), pp.418-432. 24. Geraghty, P.B., Attwood, D., Collett, J.H., Sharma, H. and Dandiker, Y., 1997. An investigation of the parameters influencing the bioadhesive properties of Myverol 18–99/water gels. Biomaterials, 18(1), pp.63-67. 25. Am Ende, M.T. and Peppas, N.A., 1997. Transport of ionizable drugs and proteins in crosslinked poly (acrylic acid) and poly (acrylic acid-co-2-hydroxyethyl methacrylate) hydrogels. II. Diffusion and release studies. Journal of controlled release, 48(1), pp.47-56. 26. Vasir, J. K., Tambwekar, K., & Garg, S. (2003). Bioadhesive microspheres as a controlleddrug delivery system. International journal of pharmaceutics, 255(1-2), 1332 27. Roy, S. and Prabhakar, B., 2010. Bioadhesive polymeric platforms for transmucosal drug delivery systems–a review. Tropical Journal of Pharmaceutical Research, 9(1)..
Scene 14 (10m 21s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 37 28. Singh, P. and Tibrewal, R., 2017. MUCOADHESIVE AND MICROSPHERE: A SHORT REVIEW. J International Journal of Medical. 29. Nagai, T. and Konishi, R., 1987. Buccal/gingival drug delivery systems. Journal of Controlled Release, 6(1), pp.353-360. 30. Sayın, B., Somavarapu, S., Li, X.W., Thanou, M., Sesardic, D., Alpar, H.O. and Şenel, S.E.V.D.A., 2008. Mono-N-carboxymethyl chitosan (MCC) and N-trimethyl chitosan (TMC) nanoparticles for non-invasive vaccine delivery. International journal of pharmaceutics, 363(1-2), pp.139-148. 31. Ilango, R., Kavimani, S., Mullaicharam, A.R. and Jayakar, B., 1997. In-vitro studies on buccal strips of glibenclamide using chitosan. Indian Journal of Pharmaceutical Sciences, 59(5), pp.232- 235. 32. Anders, R. and Merkle, H.P., 1989. Evaluation of laminated muco-adhesive patches for buccal drug delivery. International Journal of Pharmaceutics, 49(3), pp.231-240. 33. Woolfson, A.D., McCafferty, D.F. and Moss, G.P., 1998. Development and characterisation of a moisture-activated bioadhesive drug delivery system for percutaneous local anaesthesia. International Journal of Pharmaceutics, 169(1), pp.83-94. 34. Salamat-Miller, N., Chittchang, M. and Johnston, T.P., 2005. The use of mucoadhesive polymers in buccal drug delivery. Advanced drug delivery reviews, 57(11), pp.1666-1691. 35. Ugwoke, M.I., Agu, R.U., Verbeke, N. and Kinget, R., 2005. Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Advanced drug delivery reviews, 57(11), pp.1640-1665. 36. Ludwig, A., 2005. The use of mucoadhesive polymers in ocular drug delivery. Advanced drug delivery reviews, 57(11), pp.1595-1639..
Scene 15 (11m 26s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 38 37. Valenta, C., 2005. The use of mucoadhesive polymers in vaginal delivery. Advanced drug delivery reviews, 57(11), pp.1692-1712. 38. Edsman, K. and Hägerström, H., 2005. Pharmaceutical applications of mucoadhesion for the non‐ oral routes. Journal of pharmacy and pharmacology, 57(1), pp.3-22. 39. Nagai, T. and Konishi, R., 1987. Buccal/gingival drug delivery systems. journal of Controlled Release, 6(1), pp.353-360. 40. Sayın, B., Somavarapu, S., Li, X.W., Thanou, M., Sesardic, D., Alpar, H.O. and Şenel, S.E.V.D.A., 2008. Mono-N-carboxymethyl chitosan (MCC) and N-trimethyl chitosan (TMC) nanoparticles for non-invasive vaccine delivery. International journal of pharmaceutics, 363(1-2), pp.139-148. 41. Petelin, M., Pavlica, Z., Bizimoska, S. and Šentjurc, M., 2004. In vivo study of different ointments for drug delivery into oral mucosa by EPR oximetry. International journal of pharmaceutics, 270(1-2), pp.83-91. 42. Rossi, S., Marciello, M., Bonferoni, M.C., Ferrari, F., Sandri, G., Dacarro, C., Grisoli, P. and Caramella, C.A.R.L.A., 2010. Thermally sensitive gels based on chitosan derivatives for the treatment of oral mucositis. European Journal of Pharmaceutics and Biopharmaceutics, 74(2), pp.248-254. 43. Nafee, N.A., Ismail, F.A., Boraie, N.A. and Mortada, L.M., 2003. Mucoadhesive buccal patches of miconazole nitrate: in vitro/in vivo performance and effect of ageing. International journal of pharmaceutics, 264(1-2), pp.1-14. 44. Jain, A.K., Khar, R.K., Ahmed, F.J. and Diwan, P.V., 2008. Effective insulin delivery using starch nanoparticles as a potential trans-nasal mucoadhesive carrier. European Journal of Pharmaceutics and Biopharmaceutics, 69(2), pp.426-435..
Scene 16 (12m 32s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 39 45. Wu, J., Wei, W., Wang, L.Y., Su, Z.G. and Ma, G.H., 2007. A thermosensitive hydrogel based on quaternized chitosan and poly (ethylene glycol) for nasal drug delivery system. Biomaterials, 28(13), pp.2220-2232 46. Luppi, B., Bigucci, F., Abruzzo, A., Corace, G., Cerchiara, T. and Zecchi, V., 2010. Freeze- dried chitosan/pectin nasal inserts for antipsychotic drug delivery. European Journal of Pharmaceutics and Biopharmaceutics, 75(3), pp.381-387. 47. Luppi, B., Bigucci, F., Abruzzo, A., Corace, G., Cerchiara, T. and Zecchi, V., 2010. Freeze- dried chitosan/pectin nasal inserts for antipsychotic drug delivery. European Journal of Pharmaceutics and Biopharmaceutics, 75(3), pp.381-387. 48. Donnelly, R.F., McCarron, P.A., Zawislak, A.A. and Woolfson, A.D., 2006. Design and physicochemical characterisation of a bioadhesive patch for dose-controlled topical delivery of imiquimod. International journal of pharmaceutics, 307(2), pp.318-325. 49. Panicker, P.S. and Sivakumar, V., 2016. Measurement of bioadhesive strength of mucoadhesive buccal patches: Design of an in vitro assembly. International Journal of Pharmacy and Pharmaceutical Analysis, 2, pp.1-6 50. Smart, J.D., Kellaway, I.W. and Worthington, H.E.C., 1984. An in‐ vitro investigation of mucosa‐ adhesive materials for use in controlled drug delivery. Journal of Pharmacy and Pharmacology, 36(5), pp.295-299. 51. Parmar, H.K., Pandya, K.K., Pardasani, L.J., Panchal, V.S. and Tandel, T., 2017. A systematic review on mucoadhesive drug delivery system. World J Pharm Res, 6(9), pp.337-66. 52. Junginger, H.E., Hoogstraate, J.A. and Verhoef, J.C., 1999. Recent advances in buccal drug delivery and absorption—in vitro and in vivo studies. Journal of controlled release, 62(1-2), pp.149-159. 53. Wise, D.L., 2000. Handbook of pharmaceutical controlled release technology. CRC press..
Scene 17 (13m 37s)
Mucoadhesive Drug Delivery System: An Overview of Mechanism, Materials and Recent Advances INSTITUTE OF PHARMACY AND RESEARCH, BADNERA, AMRAVATI Page 40 54. Chandra Mohan, K. and Ravikumar, K., 1995. Ondansetron hydrochloride: a competitive serotonin 5-HT3 receptor blocker. Acta Crystallographica Section C: Crystal Structure Communications, 51(12), pp.2627-2629. 55. Palermo, A., Napoli, N., Manfrini, S., Lauria, A., Strollo, R. and Pozzilli, P., 2011. Buccal spray insulin in subjects with impaired glucose tolerance: the prevoral study. Diabetes, Obesity and Metabolism, 13(1), pp.42-46. 56. Battino, M., Ferreiro, M.S., Fattorini, D. and Bullon, P., 2002. In vitro antioxidant activities of mouthrinses and their components. Journal of clinical periodontology, 29(5), pp.462-467. 57. Peppas, N.A. and Sahlin, J.J., 1996. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials, 17(16), pp.1553-1561. 58. Ortega, K.L., Rezende, N.P.M., Araújo, N.S.D. and Magalhães, M.H.C.G., 2007. Effect of a topical antimicrobial paste on healing after extraction of molars in HIV positive patients: randomised controlled clinical trial. British Journal of Oral and Maxillofacial Surgery, 45(1), pp.27-29. 59. Ortega, K.L., Rezende, N.P.M., Araújo, N.S.D. and Magalhães, M.H.C.G., 2007. Effect of a topical antimicrobial paste on healing after extraction of molars in HIV positive patients: randomised controlled clinical trial. British Journal of Oral and Maxillofacial Surgery, 45(1), pp.27-29..