Page 1 (0s)
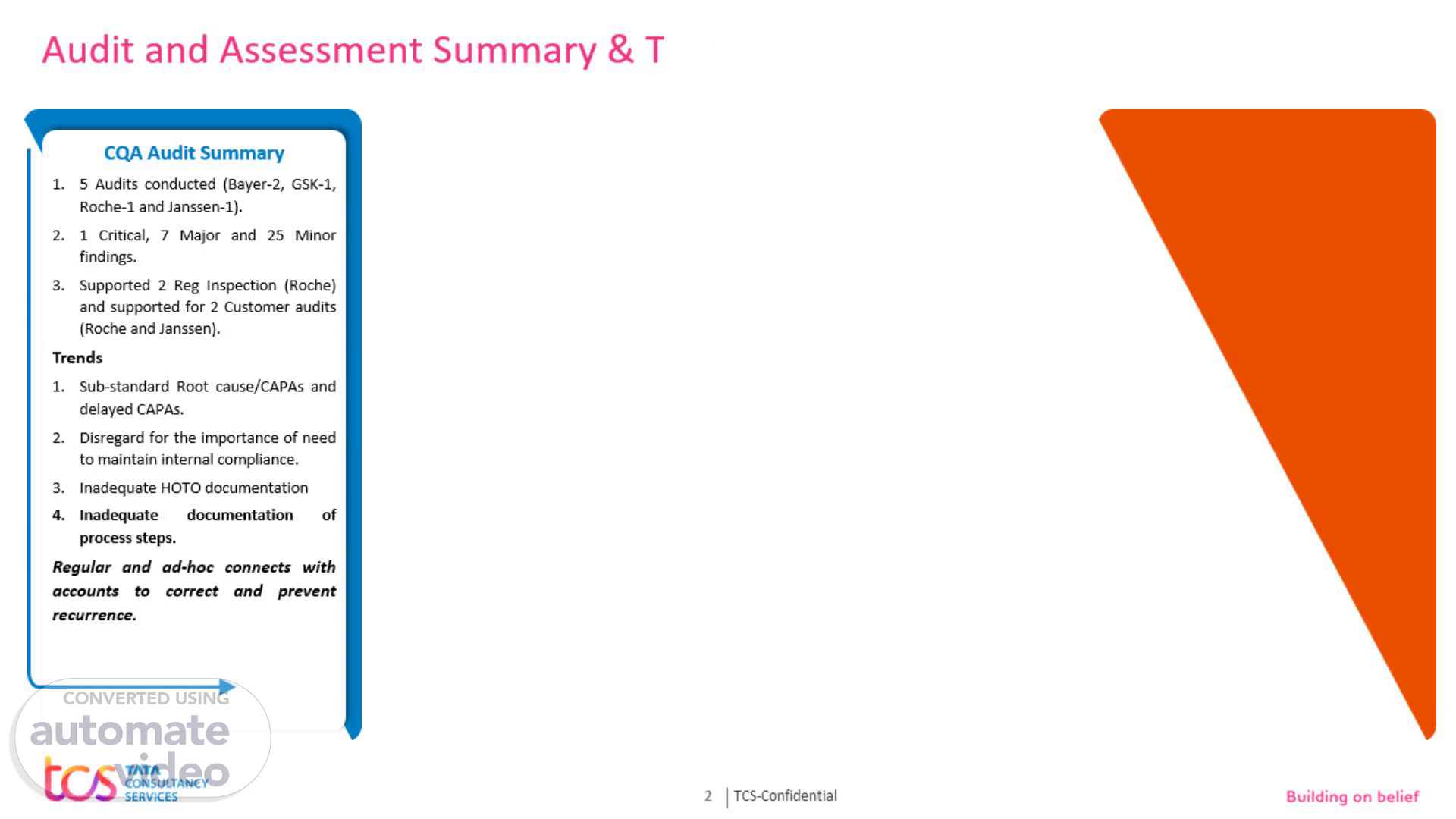
Audit and Assessment Summary & Trends. CQA Audit Summary 5 Audits conducted (Bayer-2, GSK-1, Roche-1 and Janssen-1). 1 Critical, 7 Major and 25 Minor findings. Supported 2 Reg Inspection (Roche) and supported for 2 Customer audits (Roche and Janssen). Trends Sub-standard Root cause/CAPAs and delayed CAPAs. Disregard for the importance of need to maintain internal compliance. Inadequate HOTO documentation Inadequate documentation of process steps. Regular and ad-hoc connects with accounts to correct and prevent recurrence..
Page 2 (43s)
[image] A person standing in front of a yellow wall Description automatically generated.
Page 3 (1m 38s)
[image] A person holding a smiley face and stars Description automatically generated.
Page 4 (2m 37s)
Account Conceptualization Stage Involvement GSK Guidance Shared on: Need for oversight of training, clarity on execution, reference materials easy availability – both TCS and Vendor associates Documentation of start of tasks without SOPs RACI on SOPs/Guidance materials to match TCS capability Bayer TCS Inspection readiness and joint QA committee idea shared Bayer –TCS Joint QAC has got created Training compliance checks before on-boarding on newer tasks Eli Lilly Recurring calls which resulted in fast track of Compliance to creation of role specific Training plans Process manuals in the absence of SOPs SMEs inputs considered.