Scene 1 (0s)
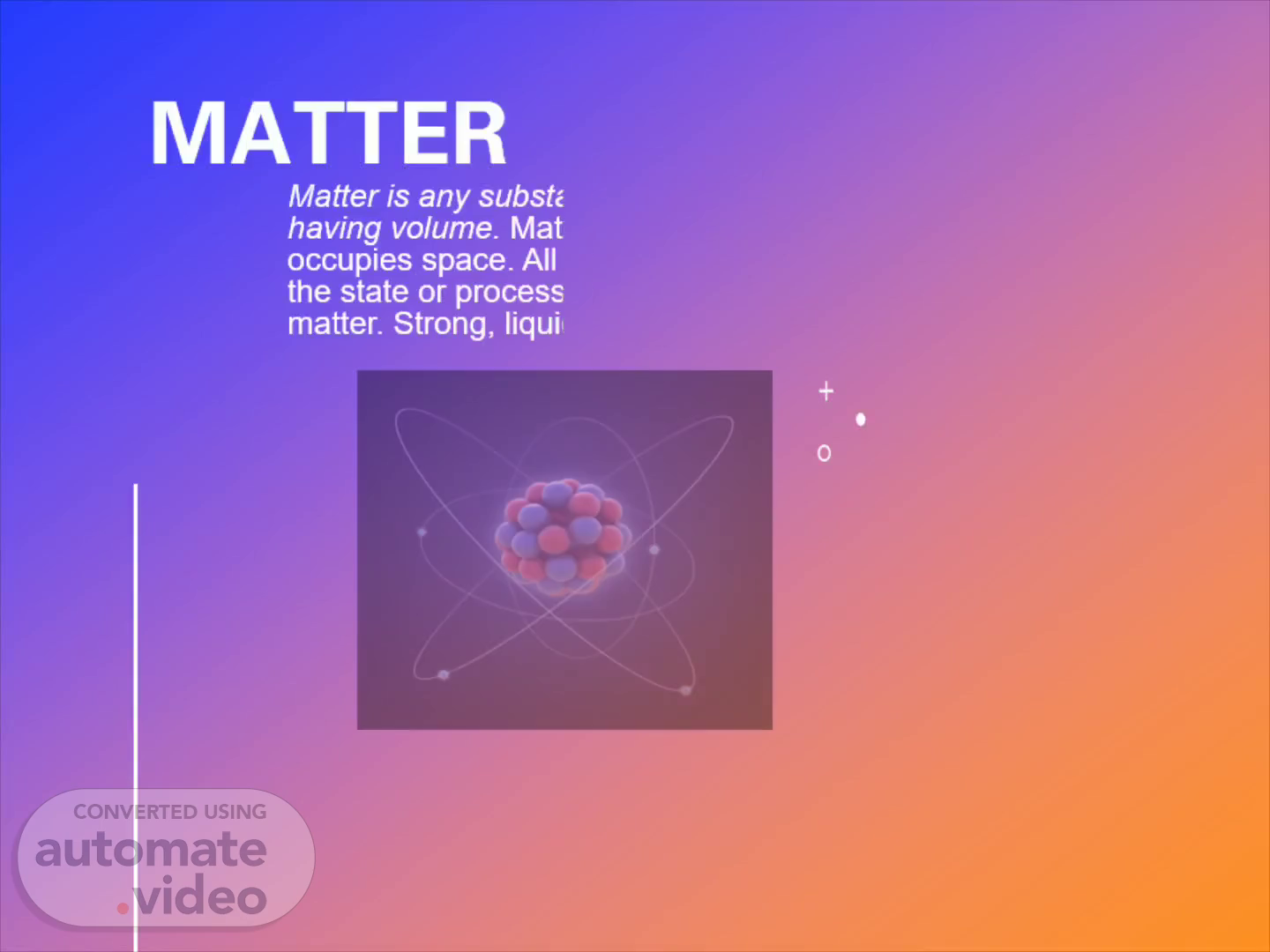
matter. abstract. Matter is any substance that has mass and takes up space by having volume . Matter is described as something that has mass and occupies space. All physical structures are made up of matter, and the state or process of matter is an easily observed property of matter. Strong, liquid, and gas are the three basic states of matter.
Scene 2 (20s)
Properties of matter. Properties of Matter Are these properties determined without changingthe identity of the No Chemical Properties How does the substance reaction to the presence of • Air • Acid Base Water Other chemicals Yes substance? No Physical Properties Yes Does the properties depend on amount of substance? Intensive Physical Property • Color Melting point Boiling point • Density Extensive Physical Property Mass Volume Length Shape.
Scene 3 (33s)
Physical Properties of Matter. Matter is made up of tiny particles called atoms and can be represented or explained as something that takes up space. It must display both the mass and volume properties . Properties are the characteristics that enable us to differentiate one material from another. A physical property is an attribute of matter that is independent of its chemical composition. Density, colour , hardness, melting and boiling points, and electrical conductivity are all examples of physical properties. Any characteristic that can be measured, such as an object’s density, colour , mass, volume, length, malleability, melting point, hardness, odour , temperature, and more, are considered properties of matter ..
Scene 4 (1m 9s)
Intensive and Extensive Properties of Matter. Intensive and Extensive Properties Intensive properties do not depend on the amount Of matter in a sample. Extensive properties depend on how much matter a sample contains. Temperature Concentration Boiling Point Luster Weight Volume Length Entropy sciencenotes.org.
Scene 5 (1m 30s)
Intensive properties of matter – An intensive property is a bulk property, which means it is a system’s local physical property that is independent of the system’s size or volume of material. Intensive properties are those that are independent of the amount of matter present. Pressure and temperature, for example, are intensive properties . Extensive property of matter – A property that is dependent on the amount of matter in a sample is known as an extensive property. Extensive properties include mass and volume. The scale of the system or the volume of matter in it determines the extensive property of the system. Extensive properties are those in which the value of a system’s property is equal to the sum of the values for the parts of the system..
Scene 6 (2m 2s)
Chemical Properties of Matter. changes i dity F I a a b i I ity Reactivity Toxic it y.
Scene 7 (2m 24s)
Reactivity – The tendency of matter to combine chemically with other substances is known as reactivity. Certain materials are highly reactive, whereas others are extremely inactive. Potassium, for example, is extremely reactive, even in the presence of water. A pea-sized piece of potassium reacts explosively when combined with a small volume of water . Flammability – The tendency of matter to burn is referred to as flammability. As matter burns, it reacts with oxygen and transforms into various substances. A flammable matter is anything like wood . Toxicity – Toxicity refers to the extent to which a chemical element or a combination of chemicals may harm an organism . Acidity – A substance’s ability to react with an acid is a chemical property. Some metals form compounds when they react with different acids. Acids react with bases to create water, which neutralizes the acid..
Scene 8 (2m 59s)
there are 4 states of matter. Solid - solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity (as in rigid bodies) and resistance to a force applied to the surfa ce..
Scene 9 (3m 41s)
Plasma. plasma , in physics , an electrically conducting medium in which there are roughly equal numbers of positively and negatively charged particles , produced when the atoms in a gas become ionized. It is sometimes referred to as the fourth state of matter , distinct from the solid , liquid , and gaseous states . The negative charge is usually carried by electrons , each of which has one unit of negative charge. The positive charge is typically carried by atoms or molecules that are missing those same electrons. In some rare but interesting cases, electrons missing from one type of atom or molecule become attached to another component, resulting in a plasma containing both positive and negative ions. The most extreme case of this type occurs when small but macroscopic dust particles become charged in a state referred to as a dusty plasma , The uniqueness of the plasma state is due to the importance of electric and magnetic forces that act on a plasma in addition to such forces as gravity that affect all forms of matter. Since these electromagnetic forces can act at large distances, a plasma will act collectively much like a fluid even when the particles seldom collide with one another..
Scene 10 (4m 39s)
5 • Dark Energy • Dark Matter Ordinary Matter. percentage of matter in the universe.
Scene 11 (4m 47s)
Dark matter. .. The term dark matter was coined in 1933 by Fritz Zwicky of the California Institute of Technology to describe the unseen matter that must dominate one feature of the universe—the Coma Galaxy Cluster..
Scene 12 (5m 30s)
Antimatter particles carry the same charge as matter particles, but of opposite sign. That is, an antiproton is negatively charged and an antielectron ( positron ) is positively charged. Neutrons do not carry a net charge, but their constituent quarks do. Protons and neutrons have a baryon number of +1, while antiprotons and antineutrons have a baryon number of –1. Similarly, electrons have a lepton number of +1, while that of positrons is –1. When a particle and its corresponding antiparticle collide, they are both converted into energy.
Scene 13 (6m 18s)
Some of the measurement of matter. Quantity Unit Symbol Temperature kelvin K Length meter M Mass Kilogram Kg weight Newton N Volume Cubic Meter m–3 Density Kilogram per cubic meter kg m–3 Pressure Pascal pa.
Scene 14 (6m 30s)
Fundamental particles of matter Electron The electron is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure Photons , electromagnetic force, leptons and quarks, all distances. Gravitons (hypothetical), force of gravity, leptons and quarks, all distances ... Neutron- Neutron A proton is made of two up quarks and one down quark, while the neutron is made of two down quarks and one up quark. These commonly bind together into an atomic ....
Scene 15 (7m 20s)
Muon - A muon is an elementary particle similar to the electron, with an electric charge of −1 e and a spin of 1⁄2, but with a much greater mass. It is classified as a lepton. As with other leptons, the muon is not thought to be composed of any simpler particles; that is, it is a fundamental particle.