Scene 1 (0s)
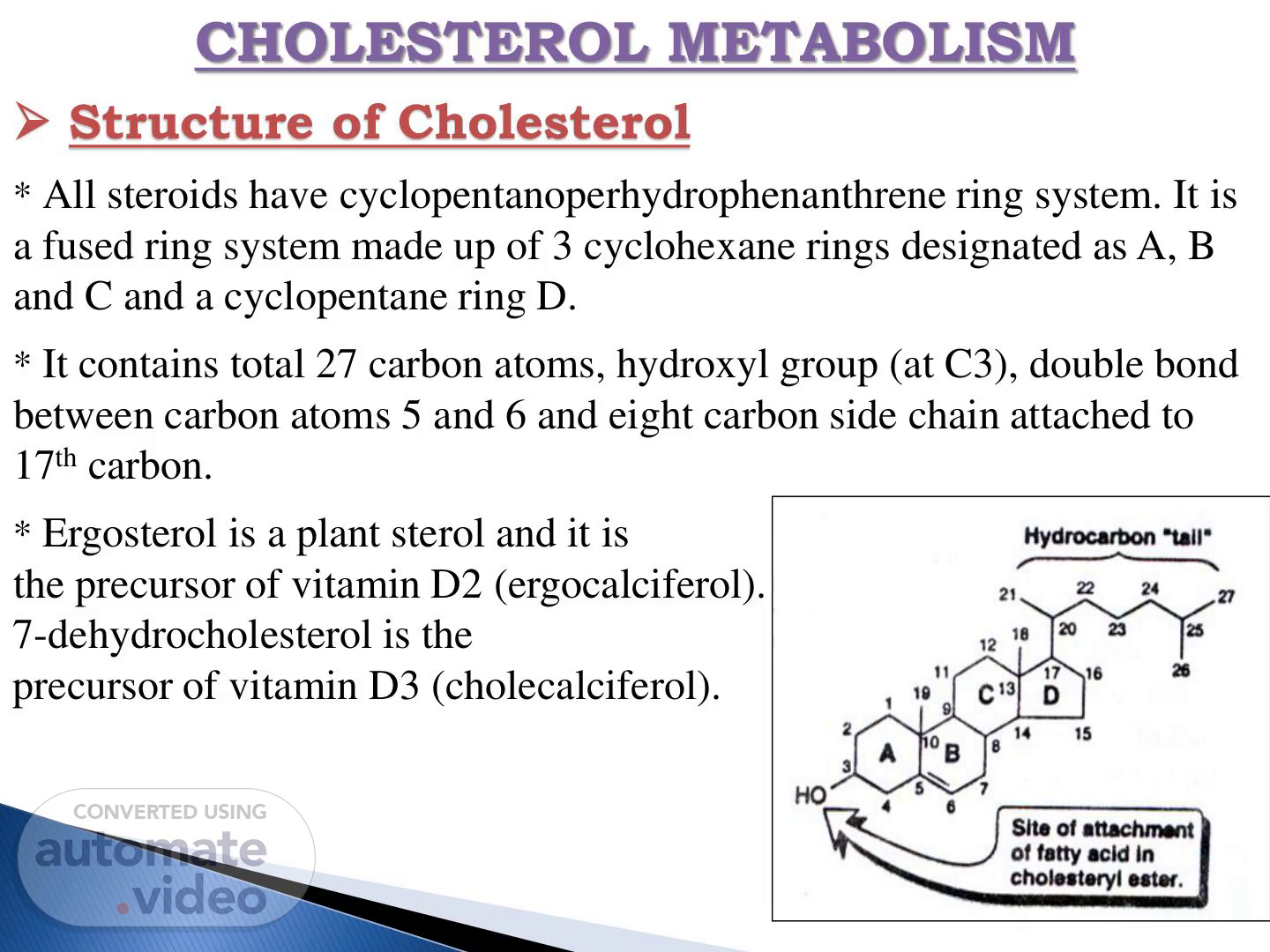
CHOLESTEROL METABOLISM ➢ Structure of Cholesterol * All steroids have cyclopentanoperhydrophenanthrene ring system. It is a fused ring system made up of 3 cyclohexane rings designated as A, B and C and a cyclopentane ring D. * It contains total 27 carbon atoms, hydroxyl group (at C3), double bond between carbon atoms 5 and 6 and eight carbon side chain attached to 17th carbon. * Ergosterol is a plant sterol and it is the precursor of vitamin D2 (ergocalciferol). 7-dehydrocholesterol is the precursor of vitamin D3 (cholecalciferol)..
Scene 2 (22s)
[Audio] The primary source of cholesterol is animal products such as eggs, meat, brain tissue, and liver oils. Most people obtain their daily dose of cholesterol from these sources. The human body synthesizes a significant portion of its cholesterol needs, with an average daily intake of around 0.3 grams. However, this amount varies greatly depending on individual diets and lifestyles. The absorption and transport of cholesterol involve several key components. Dietary cholesterol is absorbed from the intestines using bile and pancreatic juices. The absorbed cholesterol forms chylomicrons, which are carried by the bloodstream to the liver via chylomicron remnants. Once in the liver, cholesterol is distributed throughout the body via various lipoproteins, including low-density lipoprotein (LDL) and high-density lipoprotein (HDL). LDL transports cholesterol from the liver to peripheral tissues, while HDL carries it back to the liver for further processing. To maintain optimal health, understanding how cholesterol is sourced, absorbed, and transported is essential..
Scene 3 (1m 41s)
[Audio] The process of esterification of cholesterol involves two main steps within cells and one step in plasma. In cells, there are two types of reactions involved in this process. Firstly, the enzyme acyl-CoA:cholesterol O-acyltransferase (ACAT) catalyzes the reaction between cholesterol hydroxyl group and acyl-CoA resulting in the formation of cholesterol ester. Secondly, the enzyme lecithin cholesterol acyltransferase (LCAT) plays a role in the esterification of cholesterol in plasma. LCAT facilitates the conversion of cholesterol hydroxyl group into cholesterol ester when it reacts with lecithin. This results in the formation of cholesterol ester and lysolecithin. Outside of cells, the synthesis of cholesterol also occurs through various pathways. These include the production of acetyl-CoA, which serves as the starting material for all cholesterol synthesis. The synthesis of cholesterol itself occurs in multiple stages involving different enzymes such as HMG-CoA reductase. These enzymes facilitate the conversion of acetyl-CoA into cholesterol through a series of chemical reactions. The final product of these reactions is cholesterol, which is then transported out of the cell and distributed throughout the body. The carbon atoms of cholesterol are ultimately derived from acetyl-CoA. Therefore, the synthesis of cholesterol is dependent on the availability of acetyl-CoA. Overall, the biosynthesis of cholesterol is a complex process involving multiple enzymes and pathways. It requires the coordinated effort of various cellular components to produce the final product. The synthesis of cholesterol is essential for maintaining proper cellular function and overall health. It plays a critical role in the production of steroid hormones and bile acids. Furthermore, the regulation of cholesterol synthesis is tightly controlled by feedback mechanisms to maintain homeostasis. Any disruptions to this process can lead to various diseases and disorders. Therefore, understanding the biosynthesis of cholesterol is crucial for appreciating its importance in human physiology. It provides valuable insights into the underlying mechanisms of disease and allows for the development of targeted therapies. Moreover, research into the biosynthesis of cholesterol has significant implications for the treatment of various diseases. For instance, the inhibition of HMG-CoA reductase has been shown to be effective in reducing cholesterol levels. Similarly, the study of cholesterol esterification has led to the development of new treatments for certain diseases. In conclusion, the biosynthesis of cholesterol is a fascinating and complex process that continues to be the subject of ongoing research. It holds great promise for advancing our understanding of human physiology and developing novel therapeutic strategies. Ultimately, further research into the biosynthesis of cholesterol will be essential for unlocking its full potential. By doing so, we can gain a deeper appreciation for the intricate mechanisms that govern this process. And, more importantly, develop innovative solutions to address the many challenges associated with cholesterol metabolism. We'll explore the key players involved, the stages of synthesis, and the regulatory mechanisms that control this process. We'll also examine the consequences of disrupting this process and discuss the potential applications of this knowledge. Through this journey, we'll uncover the intricacies of cholesterol synthesis and reveal its significance in human physiology. And, ultimately, we'll discover the exciting possibilities that await us in the realm of cholesterol research. Now, let's dive into the world of cholesterol synthesis and explore its wonders. We'll begin by examining the process of esterification of cholesterol, which is a critical step in the synthesis of this vital molecule. As we delve into the details of esterification, we'll see how it sets the stage for the rest of the synthesis process. We'll also explore the various factors that influence the rate of esterification and how they impact the overall synthesis of cholesterol. Furthermore, we'll investigate the different pathways involved in cholesterol synthesis.
Scene 4 (6m 30s)
[Audio] The process begins with Acetyl CoA, which is converted into Acetoacetyl CoA by thiolase. This compound then reacts with another molecule of Acetyl CoA to form HMG-CoA. HMG-CoA is then reduced to Mevalonate by the enzyme HMG-CoA reductase. Mevalonate is a critical intermediate in the biosynthesis of cholesterol. It is first converted into P-hydroxy-p-methyl glutaryl-CoA, also known as HMG-CoA, through a series of reactions involving the enzymes synthetase and NADPH. This compound is then used to synthesize squalene, lanosterol, and desmosterol, which are all precursors to cholesterol. The final step involves the conversion of these compounds into cholesterol..
Scene 5 (7m 20s)
[Audio] HMG-CoA reductase is the key enzyme in cholesterol biosynthesis. The enzyme exists in two forms: a phosphorylated inactive form and a dephosphorylated active form. Phosphorylation is catalyzed by a specific reductase kinase and dephosphorylation by a protein phosphatase. HMG-CoA reductase is activated by insulin and inhibited by glucagon. During starvation, it directs acetyl CoA to the formation of ketone bodies..
Scene 6 (7m 53s)
[Audio] The breakdown of fats occurs when glucagon is present. When glucagon is not present, the breakdown of fats does not occur. Glucagon stimulates the breakdown of fats by activating hormone-sensitive lipase. Hormone-sensitive lipase breaks down triglycerides into fatty acids and glycerol. These fatty acids can then be used for energy production or stored as fat. Insulin inhibits this process. Insulin blocks the action of hormone-sensitive lipase. Without insulin, the breakdown of fats would continue unabated. However, with insulin's presence, the breakdown of fats slows down. Insulin also activates the enzyme HMG-CoA reductase which is involved in the synthesis of cholesterol. In contrast, insulin inhibits the activity of this enzyme. The regulation of cholesterol synthesis involves the interplay between glucagon and insulin. Glucagon activates HMG-CoA reductase while insulin inhibits it. This interplay results in changes in the levels of ATP, KMG-C0A, I-LMG-C0A, and other molecules involved in the process..
Scene 7 (9m 10s)
[Audio] The process of regulating cholesterol levels in cells is complex and involves multiple pathways. One key mechanism is the uptake of cholesterol-containing lipoproteins by specific receptors. This receptor-mediated uptake occurs when the lipoprotein binds to the LDL receptor on the surface of the cell. The LDL receptor recognizes the ligand and triggers a signaling cascade that ultimately leads to the internalization of the lipoprotein. Once the lipoprotein is internalized, it is then processed and either degraded or stored for future use. The non-receptor mediated uptake of cholesterol-containing lipoproteins also plays a significant role in regulating cholesterol levels. This type of uptake occurs when the lipoprotein interacts with the cell membrane and is taken up by the cell through a non-specific binding site. Free cholesterol can also be taken into the cell from cholesterol-rich lipoproteins at the cell membrane. Furthermore, the synthesis of cholesterol within the cell is another critical factor in regulating cholesterol levels. Cholesterol esters can be broken down through the action of the enzyme cholesterol ester hydrolase. The breakdown of these esters releases free cholesterol into the cell, where it can be used or stored. The regulation of cholesterol levels in cells is influenced by multiple factors, including the uptake of cholesterol-containing lipoproteins, the synthesis of cholesterol, and the breakdown of cholesterol esters..
Scene 8 (10m 49s)
[Audio] The process of cholesterol reduction involves several key factors. The efflux of cholesterol from the cell membrane to lipoproteins with low cholesterol potential, such as high-density lipoprotein (HDL), is an essential mechanism. This process is promoted by lecithin cholesterol acyltransferase (LCAT). Furthermore, cholesterol can be esterified by acyl-CoA cholesterol acetyltransferase (ACAT) and used for the synthesis of other steroids, including hormones and bile acids, in the liver. These processes collectively contribute to reducing the influx of cholesterol into tissues, thereby maintaining a healthy balance in the body..
Scene 9 (11m 35s)
[Audio] The liver plays a crucial role in eliminating cholesterol from the body. The liver converts approximately half of the ingested cholesterol into bile acids. The liver produces bile acids through oxidation, resulting in cholic acid and deoxycholic acid. The liver also converts some cholesterol into neutral sterols, such as coprostanol, which is produced by bacteria in the intestines. The liver's ability to eliminate cholesterol is essential for maintaining overall health and regulating cholesterol levels in the body..
Scene 10 (12m 8s)
[Audio] Cholesterol is a type of lipid that plays a crucial role in our body as it serves as the precursor of other steroids. This means that cholesterol acts as the starting material for the production of various types of hormones and other chemicals in our body. One of the important functions of cholesterol is its role as the parent substance of bile acids. Bile acids are essential for the digestion of fats in our body. So, cholesterol forms the building block for these bile acids to be made. In addition to this, cholesterol also plays a vital role in the production of steroid hormones of the adrenal cortex. These hormones are responsible for regulating various bodily functions such as metabolism and stress response. Not only that, but cholesterol also serves as the precursor for sex hormones, including estrogen and testosterone. These hormones play a key role in the development of secondary sex characteristics, as well as reproductive functions. Finally, let's not forget the role of cholesterol in the production of vitamin D3. After being dehydrogenated in the intestinal mucosa, cholesterol is converted into 7-dehydrocholesterol and then transformed into vitamin D3. Vitamin D3 is an important nutrient that helps in the absorption of calcium and phosphorus, which are crucial for strong bones and teeth. Cholesterol may have gained a bad reputation, but it actually plays a vital role in our body. The effects of cholesterol on our health are complex and multifaceted. High levels of cholesterol can lead to cardiovascular disease, while low levels can cause a range of problems. For example, very low levels of cholesterol can increase the risk of certain cancers. Low levels of cholesterol can also affect the immune system, leading to increased susceptibility to infections. Furthermore, high levels of cholesterol can lead to kidney damage and other complications. On the other hand, low levels of cholesterol can cause issues with bone health, particularly osteoporosis. It is essential to maintain healthy levels of cholesterol through diet, exercise, and lifestyle changes. Maintaining healthy levels of cholesterol requires careful monitoring and management. A balanced diet rich in fruits, vegetables, and whole grains can help lower cholesterol levels. Regular physical activity can also contribute to lowering cholesterol levels. Additionally, managing stress through relaxation techniques can help regulate cholesterol levels. Overall, understanding the role of cholesterol in our body is essential for maintaining good health..
Scene 11 (14m 55s)
[Audio] Cholesterol is a fatty substance that is essential for our body to function properly. However, its excess can lead to serious health problems, such as atherosclerosis and coronary heart diseases. Atherosclerosis is a condition where cholesterol and cholesterol esters of lipoproteins, such as LDL and VLDL, are deposited in the walls of our arteries. As a result, these arteries become narrow and hardened, leading to restricted blood flow. Research has shown a direct relationship between high levels of cholesterol, particularly LDL, and the incidence of coronary heart diseases and atherosclerosis in humans. This means that people with high cholesterol levels are at a higher risk of developing these diseases. Certain medical conditions, like diabetes and hypothyroidism, and lifestyle habits that lead to increased levels of LDL and VLDL in the blood, can also contribute to the development of atherosclerosis. Individuals with chronically high levels of these lipoproteins are more likely to experience premature or severe atherosclerosis. Not all cholesterol is bad; high levels of HDL, also known as the "good cholesterol," have been associated with a lower risk of developing coronary heart diseases. The balance between good and bad cholesterol is what matters, not just the total cholesterol level. Maintaining healthy cholesterol levels is crucial to preventing atherosclerosis and coronary heart diseases..
Scene 12 (16m 32s)
[Audio] The factors that increase the risk of coronary artery disease are numerous and varied. High cholesterol levels in the blood are a significant contributor to the development of CAD. Raised levels of low-density lipoprotein (LDL) cholesterol, often referred to as "bad" cholesterol, can lead to plaque buildup in the arteries, increasing the risk of heart attack and stroke. Lowered levels of high-density lipoprotein (HDL) cholesterol, known as "good" cholesterol, can also contribute to the development of CAD. High blood pressure is another major factor that increases the risk of CAD. The condition is characterized by elevated blood pressure readings, which can put additional strain on the heart and increase the risk of cardiovascular events such as heart attacks and strokes. Being overweight or obese is also a significant risk factor for CAD. Excess body fat can lead to insulin resistance, inflammation, and other metabolic problems that can increase the risk of developing CAD. Stress, smoking, and excessive alcohol consumption can all have negative effects on cardiovascular health. Furthermore, non and infrequent exercise can contribute to the risk of CAD by reducing physical fitness and increasing the risk of cardiovascular events. Post-menopausal women may be more susceptible to CAD due to hormonal changes that occur during menopause. These hormonal fluctuations can affect lipid profiles, leading to increased levels of LDL and decreased levels of HDL. Women who experience menopause may need to pay particular attention to their diet and lifestyle to reduce their risk of developing CAD..
Scene 13 (18m 25s)
[Audio] The effect of diet on serum cholesterol concentration is significant. A diet rich in cholesterol can lead to an increase in blood cholesterol levels and the formation of fatty deposits in the arteries. Fats rich in saturated fatty acids, such as those found in egg yolks and animal fats, tend to raise blood cholesterol levels, while fats rich in polyunsaturated fatty acids, like those found in vegetable oils, lower them. Polyunsaturated fatty acids, found in foods like cottonseed oil, corn oil, and soybean oil, stimulate the excretion of cholesterol into the intestine and promote the oxidation of cholesterol to bile acids, leading to rapid metabolism by the liver and other tissues..
Scene 14 (19m 13s)
[Audio] The synthesis of triglycerides, also known as triacylglycerols or TAG, involves the formation of these complex molecules from glycerol and fatty acids. This process occurs in two main locations: the liver and adipose tissue. In the liver, the primary function of TAG synthesis is to secrete very-low-density lipoprotein (VLDL) into the bloodstream, while in adipose tissue, it serves as a means of storing energy. The TAG synthesis process itself is achieved through the esterification of fatty acyl-CoA with glycerol-3-phosphate. Both glycerol and fatty acids must be present in their active forms for this reaction to occur. The glycerol component of TAG comes from the metabolic breakdown of glucose, either through the direct phosphorylation of glycerol by glycerol kinase or through the reduction of dihydroxyacetone phosphate (DHAP), a product of glycolysis. In adipose tissue and skeletal muscle, glycerol kinase is less active, resulting in the reliance on DHAP-derived glycerol for TAG synthesis. Conversely, in the liver and kidneys, glycerol kinase is more active, allowing for the use of glycerol from glucose metabolism. The process of TAG synthesis also involves the transfer of fatty acyl-CoA molecules to the hydroxyl groups of glycerol via specific acyl transferases. Furthermore, TAG synthesis occurs not only in the liver and adipose tissue but also in the intestinal mucosal cells through the medium-chain acyl-CoA dehydrogenase (MAG) pathway..
Scene 15 (21m 3s)
[Audio] The liver plays a critical role in lipid metabolism. Through gluconeogenesis, it synthesizes glycerol from glucose. Glycerol is then converted into glycerol-3-phosphate by the enzyme glycerol kinase. This molecule serves as a precursor for the synthesis of triglycerides. Additionally, the liver produces phosphatidylserine, which is involved in cellular membrane structure. The liver also stores glycogen, a complex carbohydrate that can be broken down into glucose when needed. Furthermore, the liver metabolizes fatty acids through beta-oxidation, producing acetyl-CoA, which can enter the citric acid cycle. The liver's metabolic functions are essential for maintaining energy homeostasis in the body..
Scene 16 (21m 52s)
[Audio] The stored triglycerides in adipose tissue undergo two main processes: lipolysis and re-esterification. Lipolysis breaks down triglycerides into free fatty acids, which can then be released into the bloodstream. Re-esterification converts these free fatty acids back into triglycerides, which can then be stored again in the adipose tissue. These two processes involve different reactants and enzymes. The result of these processes determines the amount of free fatty acids in the adipose tissue, which is the source of free fatty acids circulating in the plasma..
Scene 17 (22m 34s)
[Audio] The process of lipogenesis is crucial for storing energy in adipose tissue. During this process, triglycerides are synthesized from acyl-CoA and glycerol-3-phosphate. Acyl-CoA is formed from fatty acids, whereas glycerol-3-phosphate is created from glucose through the glycolytic pathway. In adipose tissue, these two substances are combined to produce triglycerides, which are then stored as energy reserves. On the other hand, lipolysis is the breakdown of triglycerides into fatty acids and glycerol. This process takes place in adipose tissue and involves the action of hormone-sensitive lipase. The resulting fatty acids can be re-esterified with glycerol-3-phosphate to reform triglycerides, thus completing the cycle. However, if the rate of re-esterification is insufficient, the accumulated fatty acids can diffuse into the bloodstream, leading to increased levels of free fatty acids. The continuous cycle between lipolysis and re-esterification is essential for maintaining energy homeostasis in the body. Disruptions to this balance can have significant consequences for overall health..
Scene 18 (23m 51s)
[Audio] Adipose tissue plays a critical role in energy storage. When glucose and insulin levels are high, adipose tissue absorbs more glucose from the bloodstream, resulting in increased glycolysis and lipogenesis. The process involves several key enzymes, including pyruvate dehydrodenase, acetyl CoA carboxylase, and glycerol phosphate acyl transferase. As a result, fat synthesis is stimulated, while fat breakdown is inhibited due to the action of insulin on hormone-sensitive lipase. During fasting periods, the metabolic landscape changes significantly. Insulin's opposing effects are overriden by other hormones such as glucagon, epinephrine, and norepinephrine, which stimulates lipolysis through the activation of adenyl cyclase. This results in the conversion of ATP to cyclic AMP, ultimately activating hormone-sensitive lipase and promoting fat breakdown..
Scene 19 (24m 54s)
[Audio] The terms listed on this slide represent various components involved in cellular energy production and regulation. ACTH and TSH refer to hormones produced by the pituitary gland that regulate other hormone secretion. Adrenalin and adrenaline-like substances are involved in stress responses. ATP, adenylate cyclase, and ST-AMP are key molecules in energy transfer within cells. Lipase enzymes play roles in fat metabolism. Triglycerides are a type of fat storage molecule. The presence of mitochondria and the absence of certain enzymes can affect cellular energy production. The cascade activation of one sensitive lipase indicates a specific metabolic pathway..
Scene 20 (25m 46s)
[Audio] The insulin is acting through receptors on the cells surface of adipocytes. These receptors are decreased, which may be an important reason for insulin insensitivity in diabetes. Obesity is caused by the fat content of the adipose tissue increasing to unlimited amounts, depending on the amount of excess calories taken in. This leads to obesity. High levels of plasma insulin are noticed, but the insulin receptors are decreased; and there is peripheral resistance against insulin action. When fat droplets are overloaded, the nucleus of adipose tissue cells is degraded, cell destruction occurs, and TAG becomes extracellular. Such TAG cannot be metabolically reutilized and forms the dead bulk in obese individuals. Leptin plays a role in obesity. Leptin is a small peptide secreted by adipocytes, and its receptors are present in the brain. When there is enough fat deposit, leptin secretion is stimulated. Neuropeptide-γ, a hypothalamic polypeptide, stimulates desire for carbohydrates. Leptin inhibits neuropeptide-γ secretion, and so when fat droplets are full, appetite is decreased. A defect in leptin or its receptor can lead to obesity..
Scene 21 (27m 10s)
[Audio] The liver plays a significant role in the process of fat metabolism. The liver's primary function in this process is the secretion of bile acids. Bile acids are essential for emulsifying fats and aiding in their digestion. The liver synthesizes fatty acids, triglycerides, and phospholipids which are necessary building blocks for the formation of fat molecules in the body. The liver oxidizes fatty acids by breaking them down into smaller molecules that can be used as energy sources. The liver produces lipoproteins which transport fats throughout the body. The liver produces ketone bodies which provide energy to the brain and other organs during periods of fasting or low carbohydrate intake. The liver synthesizes and excretes cholesterol which is vital for cell membrane structure and hormone production. The liver's functions in fat metabolism are critical to maintaining overall health..
Scene 22 (28m 13s)
[Audio] Prostaglandins are pharmacologically active compounds derived from arachidonic acid. These compounds are also known as prostanoids, which include prostaglandins, thrombo- xane and leukotrienes. Prostaglandins are 20-carbon hydroxyl fatty acids containing a 5-membered ring. They are classified into four main groups: A, B, E, and F, based on the number of double bonds in their side chains. For example, PGE2 contains two double bonds. Prostaglandins can be found in various tissues, including seminal fluid, seminal vesicles, lungs, brains, livers, and muscles. They perform several functions, including regulating cellular metabolism, causing vasodilation and lowering blood pressure, and playing roles in reproduction, cellular metabolism, and the regulation of smooth muscle contractions. Additionally, they have effects on the respiratory system, helping to relieve symptoms of bronchial asthma. Furthermore, prostaglandins can influence the activity of hormones like ACTH, glucagon, and adrenaline, and they can induce labor by causing uterine contractions. Overall, prostaglandins are complex compounds with multiple functions in the body..
Scene 23 (29m 37s)
[Audio] The prostaglandins increase cyclic AMP in platelets, thyroid, and lungs, but decrease it in adipose tissue. Prostaglandin E2 increases cyclic AMP in platelets, but decreases it in the liver and kidneys. Prostaglandin F2α increases cyclic AMP in the uterus and prostate, but decreases it in the liver and kidneys. Prostaglandin I2 increases cyclic AMP in the stomach and small intestine, but decreases it in the liver and kidneys. Prostaglandin J2 increases cyclic AMP in the pancreas, but decreases it in the liver and kidneys. Prostaglandin K2 increases cyclic AMP in the brain, but decreases it in the liver and kidneys. Prostaglandin M2 increases cyclic AMP in the skin, but decreases it in the liver and kidneys. Prostaglandin PGE2 increases cyclic AMP in platelets, but decreases it in the liver and kidneys. Prostaglandin PGF2α increases cyclic AMP in the uterus and prostate, but decreases it in the liver and kidneys. Prostaglandin PGI2 increases cyclic AMP in the stomach and small intestine, but decreases it in the liver and kidneys. Prostaglandin PGJ2 increases cyclic AMP in the pancreas, but decreases it in the liver and kidneys. Prostaglandin PGK2 increases cyclic AMP in the brain, but decreases it in the liver and kidneys. Prostaglandin PME2 increases cyclic AMP in the skin, but decreases it in the liver and kidneys. Prostaglandin PGE1 increases cyclic AMP in the stomach and small intestine, but decreases it in the liver and kidneys. Prostaglandin PGD2 increases cyclic AMP in the pancreas, but decreases it in the liver and kidneys. Prostaglandin PGF1α increases cyclic AMP in the uterus and prostate, but decreases it in the liver and kidneys. Prostaglandin PGJ1α increases cyclic AMP in the brain, but decreases it in the liver and kidneys. Prostaglandin PGE3 increases cyclic AMP in the skin, but decreases it in the liver and kidneys. Prostaglandin PGF3α increases cyclic AMP in the pancreas, but decreases it in the liver and kidneys. Prostaglandin PGJ3α increases cyclic AMP in the brain, but decreases it in the liver and kidneys. Prostaglandin PGE4 increases cyclic AMP in the stomach and small intestine, but decreases it in the liver and kidneys. Prostaglandin PGF4α increases cyclic AMP in the uterus and prostate, but decreases it in the liver and kidneys. Prostaglagland D2 increases cyclic AMP in the pancreas, but decreases it in the liver and kidneys. Prostaglandin E2 increases cyclic AMP in platelets, but decreases it in the liver and kidneys. Prostaglandin F2α increases cyclic AMP in the uterus and prostate, but decreases it in the liver and kidneys. Prostaglandin G2 increases cyclic AMP in the pancreas, but decreases it in the liver and kidneys. Prostaglandin H2 increases cyclic AMP in the stomach and small intestine, but decreases it in the liver and kidneys. Prostaglandin I2 increases cyclic AMP in the stomach and small intestine, but decreases it in the liver and kidneys. Prostaglandin J2 increases cyclic AMP in the pancreas, but decreases it in the liver and kidneys. Prostaglandin K2 increases cyclic AMP in the brain, but decreases it in the liver and kidneys. Prostaglandin L2 increases cyclic AMP in the skin, but decreases it in the liver and kidneys. Prostaglandin M2 increases cyclic AMP in the skin, but decreases it in the liver and kidneys. Prostaglandin N2 increases cyclic AMP in the pancreas, but decreases it in the liver and kidneys. Prostaglandin O2 increases cyclic AMP in the brain, but decreases it in the liver and kidneys. Prostaglandin PGE2 increases cyclic AMP in platelets, but decreases it in the liver and kidneys. Prostaglandin PGF2α increases cyclic AMP in the uterus and prostate, but decreases it in the liver and kidneys. Prostaglandin PGI2 increases cyclic AMP in the stomach and small intestine, but decreases it in the liver and kidneys. Prostaglandin PGJ2 increases cyclic AMP in the pancreas, but decreases it in the liver and kidneys. Prostaglandin.
Scene 24 (34m 37s)
[Audio] The leukotrienes are hydroxylated fatty acids that are derived from arachidonic acid. These compounds are produced by cells called leukocytes, platelets, and macrophages. The leukotrienes play a role in various physiological processes, including chemotaxis, inflammation, and allergic reactions. There are different types of leukotrienes, such as Leukotriene D4 and Leukotriene B4. Leukotriene D4 causes constriction of smooth muscles, resulting in narrowed pulmonary airways and coronary arteries. Leukotriene B4 attracts white blood cells like neutrophils and eosinophils to areas of inflammation..
Scene 25 (35m 21s)
[Audio] The process of arachidonic acid release from membrane phospholipids occurs when a specific enzyme called phospholipase A2 is activated. This enzyme breaks down the phospholipid molecule into two parts: one part remains attached to the cell membrane, while the other part is released as free arachidonic acid. The release of arachidonic acid triggers the synthesis of various eicosanoids, including prostaglandins, thromboxanes, and leukotrienes. These eicosanoids play a crucial role in mediating inflammation and regulating blood pressure. The regulation of arachidonic acid release involves an inhibitory protein called lipocortin, which reduces the release of arachidonic acid. Lipocortin is induced by glucocorticoid hormones, such as cortisol. When lipocortin binds to its receptor, it exerts an anti-inflammatory effect by reducing the production of prostanoids, such as prostaglandins and thromboxanes. This reduction in prostanoid production leads to decreased inflammation and lower blood pressure..
Scene 26 (36m 33s)
[Audio] The cyclooxygenase enzyme system is responsible for converting arachidonic acid into prostaglandins, such as PGI2. This process involves the conversion of arachidonic acid into prostaglandin H2, followed by its transformation into other prostaglandins like PGE1 and PGF2α. The lipooxygenase enzyme system, on the other hand, converts arachidonic acid into leukotrienes. Leukotrienes are known to be potent inhibitors of platelet aggregation and have been shown to cause bronchoconstriction in asthma patients. The effectiveness of these systems can vary depending on the specific context. For example, in inflammatory conditions, the cyclooxygenase system is often activated, leading to increased production of prostaglandins. In contrast, in certain types of cancer, the lipooxygenase system may be overactive, resulting in elevated levels of leukotrienes. The use of nonsteroidal anti-inflammatory drugs (NSAIDs) has been a long-standing approach to managing inflammation. However, recent studies suggest that steroids, which inhibit both the cyclooxygenase and lipooxygenase systems, may offer greater benefits in reducing inflammation. This is because steroids can effectively suppress the production of both prostaglandins and leukotrienes, whereas NSAIDs primarily target the cyclooxygenase system. As a result, steroids appear to be more effective anti-inflammatory agents than NSAIDs in certain situations..