Microsoft PowerPoint - FATS AND OILS 2024 [Compatibility Mode]
Scene 1 (0s)
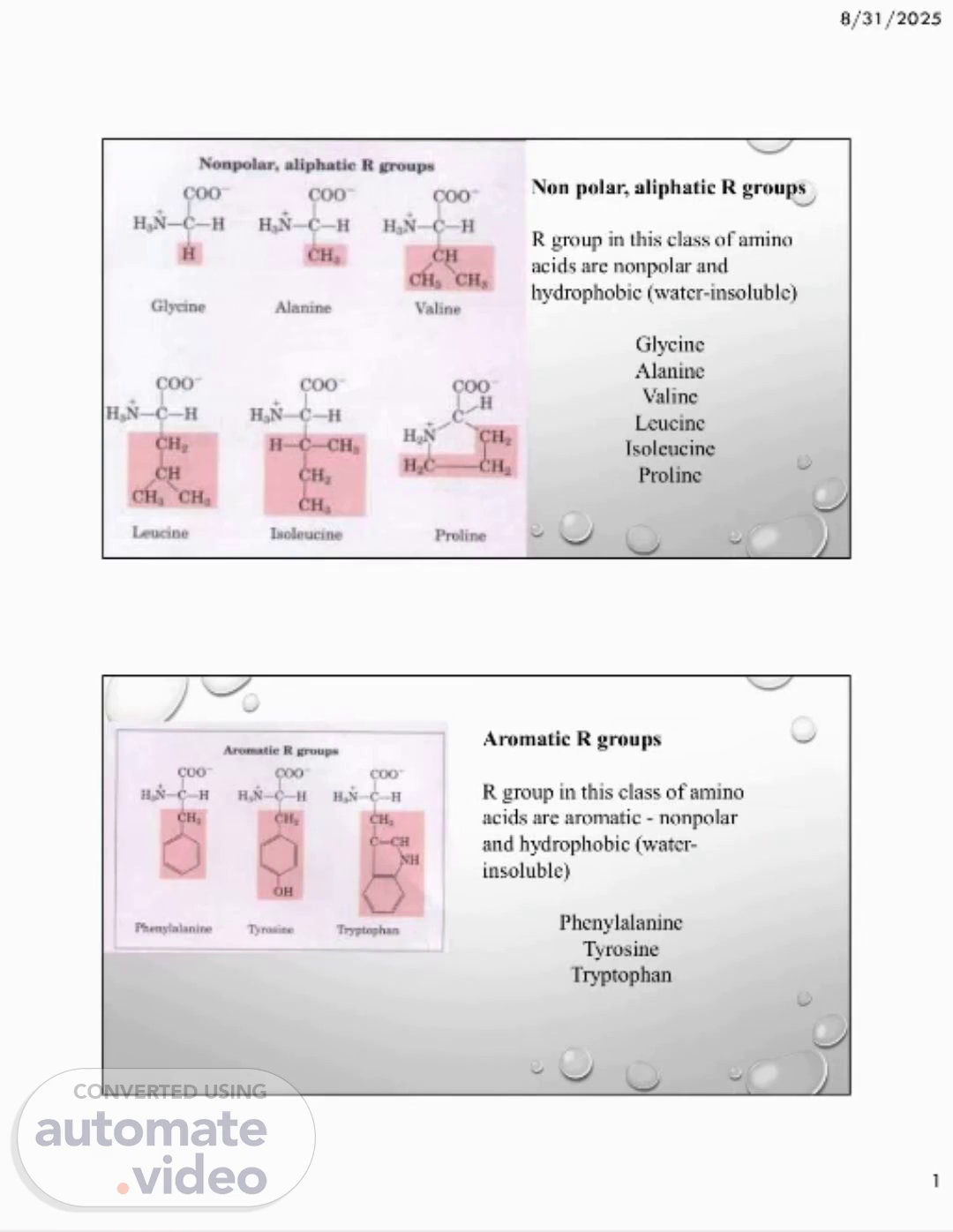
8/31/2025 1 Non polar, aliphatic R groups R group in this class of amino acids are nonpolar and hydrophobic (water-insoluble) Glycine Alanine Valine Leucine Isoleucine Proline Aromatic R groups R group in this class of amino acids are aromatic - nonpolar and hydrophobic (water- insoluble) Phenylalanine Tyrosine Tryptophan.
Scene 2 (2m 15s)
[Audio] Today, we will discuss the properties of nonpolar, aliphatic, and aromatic R groups in amino acids and their impact on solubility in water. The R groups are divided into two categories: polar and nonpolar, as shown on slide 2. Let's begin with nonpolar, aliphatic, and aromatic R groups, which are known for being hydrophobic and thus not soluble in water. This is because they consist mostly of carbon and hydrogen atoms that do not easily interact with water molecules. In contrast, polar uncharged R groups are hydrophilic and water-soluble. Examples of this group include serine, threonine, cysteine, methionine, asparagine, and glutamine, which contain oxygen and nitrogen atoms that can interact with water molecules. Moving on to positively charged R groups, we see that they are also polar and hydrophilic. These R groups have a net positive charge, leading them to be attracted to polar water molecules. Some examples of amino acids with positively charged R groups are lysine, arginine, and histidine. Understanding the properties of these R groups is crucial as they can affect the structure and function of proteins. For instance, the presence of hydrophobic R groups can result in the formation of a hydrophobic core in proteins, while polar and charged R groups may aid in the protein's ability to interact with water and other molecules. To summarize, the composition of R groups has a significant impact on the solubility of amino acids in water. Nonpolar, aliphatic, and aromatic R groups are hydrophobic and insoluble in water, while polar R groups are hydrophilic and water-soluble. Stay tuned for more information on the different groups of amino acids..
Scene 3 (4m 11s)
[Audio] We will be discussing the characteristics of nonpolar, aliphatic, and aromatic R groups found in this group of amino acids. These R groups are hydrophobic and insoluble in water. The date on the slide is 8/31/2025, which is the scheduled date of our presentation. We will now focus on the negatively charged R group, which holds a net negative charge. Moving on to the polar and hydrophilic R groups, these are water-soluble and examples include aspartate and glutamate. Thank you for listening and let's continue with our presentation..
Scene 4 (4m 49s)
[Audio] We are currently on slide 4 out of 19 in our presentation on Nonpolar, Aliphatic, and Aromatic R groups in amino acids. These groups are hydrophobic and insoluble in water, and now we will focus on the tertiary structure and the role of chain folding in the physical properties and biological function of these groups. This process is caused by interactions between the R groups, leading to the unique three-dimensional tertiary structure. This structure can be fibrous or globular, with collagen and keratin having a long, extended shape and enzymes and other proteins having a compact, rounded shape. The chain folding process is crucial because it determines the physical properties of these amino acids and also plays a significant role in their biological function. For example, collagen's fibrous structure provides strength and support for connective tissues, while enzymes' globular structure allows for precise and efficient chemical reactions. Understanding this concept is vital in the study of biochemistry. Our next slide will continue this topic. Thank you for your attention..
Scene 5 (6m 5s)
[Audio] We will be discussing the different types of R groups present in amino acids and their role in the quaternary structure. Some amino acids have nonpolar, aliphatic, and aromatic R groups that are hydrophobic and do not mix well with water. This allows them to form interactions with other molecules. Moving on, the quaternary structure consists of multiple subunits or monomers, each containing two polypeptides. These subunits are held together by interactions such as hydrogen bonding, van der Waals interactions, and ionic bonding. These interactions are essential for maintaining the stability and function of the quaternary structure. For example, the quaternary structure of hemoglobin contains four subunits, each consisting of two polypeptides with different numbers of amino acids. These interactions are crucial for the proper functioning of hemoglobin as an oxygen-carrying protein. Understanding the different types of R groups and their role in the quaternary structure is crucial in comprehending the complexity of amino acids. This concludes our presentation on amino acids. Thank you for your attention and we hope this has given you a better understanding of this important topic..
Scene 6 (7m 21s)
[Audio] Today, we will be discussing the nonpolar, aliphatic, and aromatic R groups found in certain amino acids. This is an important topic for those studying higher education, specifically in the field of biology and biochemistry. Slide number six focuses on the hydrophobic nature of these R groups, meaning they are not attracted to water and are insoluble. This important characteristic is crucial in fully understanding the structure and function of certain molecules. Let's take a closer look at two examples: human insulin and hemoglobin. Human insulin has a quaternary structure, made up of four subunits. The A chain, also known as the alpha chain, is composed of 21 amino acids while the B chain has 30 amino acids. This brings the total number of amino acids in human insulin to ... as seen on the slide. Moving on to hemoglobin, we see a similar quaternary structure, but with some differences. This molecule is a tetramer, consisting of four subunits, with one polypeptide chain per subunit. The alpha chain has 141 amino acids while the beta chain has 146. Therefore, the total number of amino acids in hemoglobin is ... as shown on the slide. Understanding the structure and composition of these molecules is crucial in understanding their functions in the human body. This knowledge will also aid in further research and advancements in the medical field. Thank you for joining us for this presentation on nonpolar, aliphatic, and aromatic R groups in amino acids. The remaining slides will further explore this fascinating topic. Thank you..
Scene 7 (9m 9s)
[Audio] In this presentation, we have discussed the basic structure of amino acids and their classification based on the polarity of their side chains. Slide number 7 provides a detailed look at the different types of R groups in amino acids, specifically the nonpolar, aliphatic, and aromatic groups known for their hydrophobic and insoluble properties. These R groups play a crucial role in the overall structure and function of proteins, including the hemoglobin molecule. Hemoglobin is composed of four protein subunits (two alpha and two beta), each of which folds into specific structural segments, such as alpha-helices. These segments create a pocket that strongly binds the heme group, allowing efficient oxygen transport in the blood. The presence of salt bridges forms a tetrameric quaternary structure, holding the four polypeptide chains together. By understanding the significance of these R groups in hemoglobin formation, we gain a better appreciation for the complex structures and vital functions of proteins in our bodies. Stay tuned for the remaining slides as we delve further into the world of amino acids. Thank you for your attention..
Scene 8 (10m 26s)
[Audio] We will be discussing the role of nonpolar, aliphatic, and aromatic R groups in amino acids. These groups are hydrophobic and insoluble in water. This slide contains an important date - August 31st, 2025, the deadline for our enzyme kinetics project. Enzymes play a crucial role in affecting reaction rates by acting as catalysts. This simple enzymatic reaction consists of four steps - enzyme, substrate, product, and a transient complex of the enzyme with the substrate, known as ES. The reaction coordinate diagram helps us visualize the energy changes in a reaction. In biological systems, energy is described in terms of free energy, known as G. This concept is important in biochemistry for understanding energy changes in a system. The diagram plots the free energy of the system against the progress of the reaction, allowing us to study the relationship between energy changes and the progress of the reaction. This concludes our discussion on the role of nonpolar, aliphatic, and aromatic R groups in enzyme kinetics. On the next slide, we will explore the factors that affect enzyme activity..
Scene 9 (11m 46s)
[Audio] We are now on slide 9 out of 19 in our presentation on nonpolar, aliphatic, and aromatic R groups in amino acids. Today, we will be discussing the role of hydrophobic and insoluble R groups in these amino acids. On this slide, we can see the date of August 31st, 2025 and the text "In the reaction S to P," which refers to the process of converting the substrate (S) into the product (P). Moving on, we will now talk about the free energy of the ground state of P compared to S. The free energy of P is lower, indicating a negative overall free energy change (ΔG) for this reaction. This means that at equilibrium, there will be more P than S. Next, we can see the energy barrier, or the energy hill, between S and P. In order for the reaction to occur, the molecules must overcome this barrier. The top of the hill is known as the transition state (TS), where the molecules have an equal chance of either decaying to the substrate or the product. The difference in energy between the ground state and the transition state is called the activation energy (ΔG‡), which is the energy required for the reaction to take place. The higher the activation energy, the slower the reaction will be, while a lower activation energy will result in a faster reaction. In summary, the rate of a reaction is directly related to the activation energy. We will now move on to slide 10, where we will delve further into this concept..
Scene 10 (13m 25s)
[Audio] This slide will discuss the properties of nonpolar, aliphatic, and aromatic R groups in certain amino acids. These groups are considered hydrophobic, meaning they are not soluble in water. Let's now explore how we can increase the rate of a chemical reaction by lowering the activation energy. The activation energy is the minimum amount of energy required for a reaction to occur. By adding a catalyst, we can reduce the activation energy and increase the reaction rate. A catalyst is a substance that helps to speed up a chemical reaction without being used up. Enzymes are a specific type of catalyst that play a significant role in biological reactions. It is important to note that enzymes will catalyze the reaction in both directions, from S to P and vice versa. Additionally, when an enzyme catalyzes a reaction, two intermediate complexes are formed, the ES complex and the EP complex. These complexes are crucial in accelerating the conversion between S and P. Let's now examine the enzyme itself. It is not consumed in the process, allowing it to catalyze reactions repeatedly. This makes enzymes highly efficient and essential for biochemical processes in living organisms. Next, we will discuss the types of enzymes and their functions on the following slide..
Scene 11 (14m 47s)
[Audio] We have now reached slide 11 where we will discuss the properties of nonpolar, aliphatic, and aromatic R groups in amino acids. These hydrophobic groups play a crucial role in biochemical processes and are insoluble in water. Next, we will explore two important terms - ES and EP complexes, which occupy valleys in the reaction coordinate diagram representing energy levels during a reaction. It is essential to note that the overall rate of a reaction is determined by the step with the highest activation energy, known as the rate-limiting step, if several steps occur in a reaction. Moving on, we will delve into Enzyme Kinetics, a powerful approach to understanding enzyme mechanisms and their function in biological systems. Through enzyme kinetics, we can determine the rate of a reaction and how it responds to changes in experimental parameters, such as the substrate concentration ([S]). This concentration plays a critical role in determining the rate of an enzyme-catalyzed reaction. Let's now move on to slide 12..
Scene 12 (15m 57s)
[Audio] In this presentation, we have discussed the role of various amino acid groups in enzyme activity and the relationship between substrate concentration and the initial rate of enzyme activity, V0. On slide 12, we can see that at lower substrate concentrations, V0 increases linearly. However, as substrate concentration increases, the rate of increase in V0 becomes smaller due to the hydrophobic and insoluble nature of certain amino acid groups. This leads to a plateau-like region close to the maximum velocity, Vmax, indicating that the enzyme is functioning at its maximum capacity. This behavior is explained by the ES complex, which determines the response of the enzyme to varying substrate concentrations. The hydrophobic and insoluble nature of certain amino acid groups plays a significant role in enzyme kinetics. Thank you for your attention and see you on our next slide for more on this topic..
Scene 13 (17m 2s)
[Audio] We are now on slide number 13 of our presentation on Nonpolar, Aliphatic, and Aromatic R groups in amino acids. Our topic for today is enzyme kinetics and its significance in enzymatic catalysis. This theory was initially proposed by Victor Henri and Wurtz in 1903, when they noticed a particular kinetic pattern in Figure. They concluded that the formation of an ES complex between an enzyme and its substrate is a crucial step in enzyme action. In 1913, Leonor Michaelis and Maud Menten expanded on this idea, suggesting that the first step in enzyme catalysis is a fast and reversible process of forming the enzyme-substrate complex. This theory has been widely accepted and has greatly enhanced our knowledge of enzymatic reactions. We have now reached the end of slide number 13. Let's continue our exploration of enzymes by moving on to the remaining slides. Thank you for your attention and I look forward to discussing slide number 14 with you..
Scene 14 (18m 8s)
[Audio] Slide number 14 discusses the role of nonpolar, aliphatic, and aromatic R groups in amino acids. These R groups are known as hydrophobic and are insoluble in water. The first step of the reaction forms the ES complex, or enzyme-substrate complex, while the slower second step breaks down this complex into the free enzyme and the reaction product P. The rate of the second step determines the overall reaction rate and is directly proportional to the concentration of ES. At low substrate concentrations, most of the enzyme is in the free form, E. As the substrate concentration increases, the equilibrium shifts towards the formation of more ES, leading to a rate proportional to the substrate concentration. Understanding this concept is important in enzyme kinetics as it highlights the significance of the second step in determining the overall reaction rate. The next slide will delve into the two forms of enzymes present in a catalyzed reaction..
Scene 15 (19m 15s)
[Audio] We will now move on to slide 15 of our presentation where we will examine the role and characteristics of nonpolar, aliphatic, and aromatic R groups in amino acids. These groups are considered hydrophobic as they are not soluble in water. The date for our discussion is August 31st, 2025. Our focus will now shift to Enzyme Kinetics. Enzymes are essential in catalyzing reactions in our bodies. The maximum initial rate of a catalyzed reaction, known as Vmax, is achieved when the enzyme is fully saturated with its substrate, with no free enzymes or minimal [E]. This saturation effect is responsible for the plateau shown in the figure on our slide. This means that further increases in substrate concentration will not impact the reaction rate. However, once the ES complex breaks down to produce the product, the enzyme can then catalyze another molecule of substrate, resulting in an increase in reaction rate. This process, known as saturation kinetics, ensures that enzymes are working efficiently and at their maximum potential. Understanding the role of nonpolar, aliphatic, and aromatic R groups in this process is crucial in enzyme kinetics and is essential to continue our discussion on the topic. Moving on to the next slide, we will delve further into the impact of these R groups in different enzymes and their functions. Let's continue our learning journey..
Scene 16 (20m 52s)
[Audio] Slide number 16 discusses the nonpolar, aliphatic, and aromatic R groups found in amino acids, which are hydrophobic and not soluble in water. Today's topic is enzyme kinetics, specifically pre-steady state and steady-state kinetics. In the pre-steady state, the enzyme is mixed with an excess of substrate, leading to the build-up of the ES complex. Once the initial period ends, the reaction reaches a steady state where the concentration of ES remains constant. We use steady-state kinetics to analyze the initial reaction rate. This method shows the effect of changing substrate concentration on the reaction rate. Most enzymes follow a rectangular hyperbolic shape in this relationship, which can be expressed mathematically by the Michaelis Menten equation. This equation allows us to calculate the rate based on substrate concentration. Understanding enzyme kinetics is essential in determining the rate and efficiency of enzymatic reactions. So, that concludes slide number 16. See you on the next slide..
Scene 17 (22m 7s)
[Audio] This powerpoint slide discusses the relationship between substrate concentration and reaction rate and how it can be expressed quantitatively through an equation. The equation includes terms such as [S] for substrate concentration, V0 for initial reaction rate, Vmax for maximum reaction rate, and Km for Michaelis constant. These terms can be measured using experimental methods. The accuracy of this equation in representing experimental observations can be evaluated by considering two scenarios: high and low substrate concentrations, as shown in Figure 17. Through analysis, we can better understand the impact of substrate concentration on reaction rate. This also helps in understanding the hydrophobic nature of nonpolar, aliphatic, and aromatic R groups in amino acids that make them insoluble in water. This knowledge contributes to our understanding of biochemistry. Let's continue to the next slide..
Scene 18 (23m 13s)
[Audio] In this slide, we will discuss the impact of nonpolar, aliphatic, and aromatic R groups on the hydrophobicity and solubility of amino acids. We will also consider the Michaelis-Menten equation and its fit with our experimental observations. When the concentration of substrate is low, the equation simplifies to V0 equals Vmax times [S]. On the other hand, when the substrate concentration is high, the equation simplifies to V0 equals Vmax. This aligns with our observations and explains the plateau seen at high substrate concentrations, where the enzyme's active sites are saturated. In conclusion, the Michaelis-Menten equation is consistent with our observations and accounts for the plateau at high substrate concentrations. Thank you for your attention. This concludes our presentation on amino acids and enzyme kinetics..
Scene 19 (24m 12s)
[Audio] As we reach the end of our presentation, we will now focus on the 19th slide. This slide discusses the nonpolar, aliphatic, and aromatic R groups found in a group of amino acids. These particular R groups are characterized as hydrophobic, meaning they are repelled by water and are insoluble in it. Moving on to another important topic, we will explore the relationship between substrate concentration and reaction rate. This relationship can be expressed quantitatively through the Michaelis-Menten equation. When the initial reaction rate (V0) is half of the maximum reaction rate (Vmax), we can derive an important numerical relationship known as the Michaelis constant (Km). This value represents the substrate concentration at which V0 is equal to half of Vmax. Understanding this concept is crucial for studying enzyme kinetics, as it helps us determine the necessary substrate concentration for a desired reaction rate. Thank you for your attention during this presentation. I hope you have gained a deeper understanding of the topics we have discussed. We will now conclude our presentation and I welcome any questions or further discussions you may have..