Scene 1 (0s)
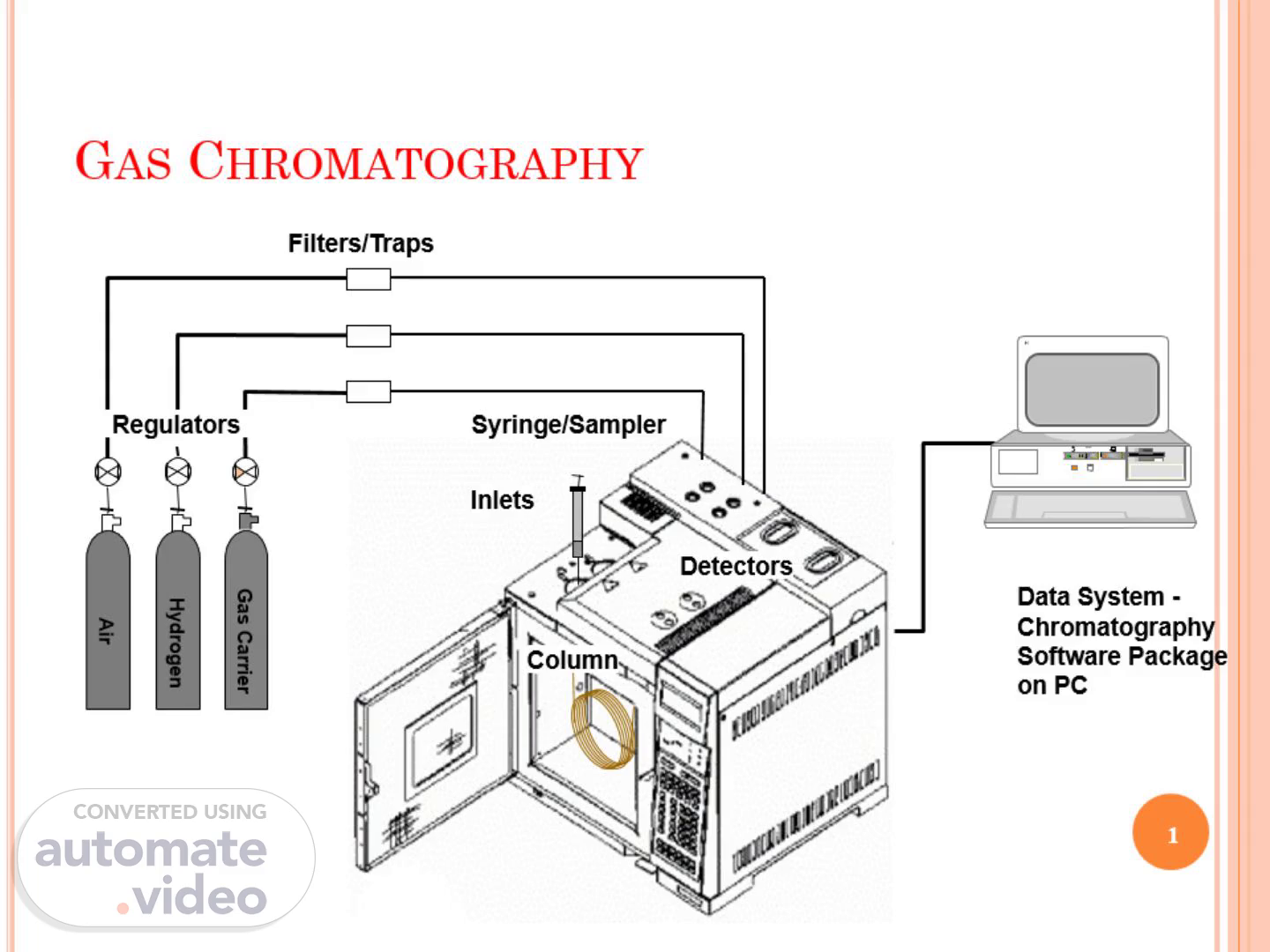
[Audio] The gas chromatography is a laboratory technique that separates, identifies, and quantifies the components of a mixture based on their boiling points. This process involves injecting a sample into a heated column where it is separated from other components. The separated components then exit the column and are detected by a detector. The resulting data is analyzed using software to determine the composition of the original mixture..
Scene 2 (25s)
[Audio] Gas chromatography is a simple and efficient method for separating gaseous and volatile samples when they are heated. Two main types of gas chromatography exist, distinguished by the type of stationary phase involved. The most common type is gas-liquid chromatography, where the stationary phase is a liquid adsorbed onto a solid surface. This type of chromatography is widely used due to the availability of various liquid stationary phases. In contrast, gas-solid chromatography uses a solid material as the stationary phase, but it is less commonly used due to the limited number of available solid stationary phases..
Scene 3 (1m 5s)
[Audio] The sample, which consists of a solute dissolved in a solvent, is first prepared. The mixture is then vaporized onto the head of a column. At this stage, the vaporized solvent and solute are carried through the column by an inert gas, known as the mobile phase. This mobile phase does not interact with the compounds of interest. Consequently, the component more soluble in the stationary phase travels slower, whereas the component less soluble in the stationary phase travels faster. This separation process takes place based on the partition coefficient of each compound, enabling us to differentiate between them..
Scene 4 (1m 41s)
[Audio] In gas chromatography, all solvent molecules pass through the column unretained. This means they do not interact with the stationary phase and are not separated from each other. On the other hand, separation occurs when the solute interacts with the stationary phase. This interaction can occur through adsorption, where the solute molecule binds to the surface of the stationary phase, or partition, where the solute molecule dissolves in the stationary phase. The type of interaction depends on whether we are referring to gas-solid chromatography or gas-liquid chromatography. In gas-solid chromatography, adsorption occurs, while in gas-liquid chromatography, partitioning takes place. Finally, different types of detectors can be used in gas chromatography, including thermal conductivity, flame ionization, thermionic, and electron capture detectors. These detectors measure various physical properties of the eluted compounds, allowing us to identify and quantify them..
Scene 5 (2m 42s)
[Audio] Compounds A and B interact with the stationary phase through intermolecular forces, such as van der Waals or dipole-dipole forces, including hydrogen bonding. Compound A interacts more strongly with the liquid stationary phase and is retained relative to compound B, which interacts weakly with the stationary phase. As a result, compound B spends more time in the gas phase and advances more rapidly through the column, resulting in a shorter retention time compared to compound A. Furthermore, compounds with similar polarity tend to elute in order of their volatility, meaning that alkanes, for instance, elute in order of increasing boiling points. Lower boiling alkanes will therefore have shorter retention times than higher boiling alkanes..
Scene 6 (3m 27s)
[Audio] The gas chromatography system utilizes a carrier gas, either nitrogen or helium, to transport the sample through the column. This carrier gas plays a crucial role in separating the components of the mixture. The injector introduces the sample into the system, while the oven provides the necessary temperature control to facilitate the separation process. The column itself is where the actual separation takes place, using the carrier gas to distribute the components evenly. Finally, the detector measures the amount of each component present in the sample, providing valuable information about the composition of the mixture..
Scene 7 (4m 3s)
[Audio] The example gas chromatography system consists of a filter and trap section, where impurities are removed from the sample gas stream. The air and hydrogen gas carrier provides the necessary gases for the analysis. The column separates the sample using the principles of gas-solid chromatography. The data system, including the chromatography software package on the PC, collects and analyzes the data. The syringe/sampler inlets, detectors, regulators, and purge valves work together to ensure accurate and reliable results..
Scene 8 (4m 35s)
[Audio] The carrier gas, also known as the mobile phase, plays a crucial role in the gas chromatography process. Its primary function is to transport the analyte through the liquid or solid stationary phase. Several types of carrier gases are commonly used, including helium, argon, nitrogen, and hydrogen. Each of these gases has its own advantages and disadvantages. For instance, hydrogen has good thermal conductivity, but it can react with unsaturated compounds. Helium, on the other hand, has excellent thermal conductivity, but it is more expensive than some other options. Nitrogen, although less expensive, has reduced sensitivity due to its lower thermal conductivity. When selecting a carrier gas, factors such as inertness, suitability for the detector, high purity, ease of availability, and cost must be considered. Moreover, the carrier gas should not pose a risk of fire and should provide optimal column performance..
Scene 9 (5m 34s)
[Audio] For optimum column efficiency, it's essential to introduce the sample into the column as a plug of vapor. Slow injection of a large sample can lead to band broadening and loss of resolution. To achieve this, a GC syringe penetrates a septum to inject the sample into the vaporization chamber. This process involves instant vaporization of the sample, typically occurring at a temperature 50 degrees Celsius higher than its boiling point. The carrier gas then transports the sample into the head of the column. The purge valve plays a crucial role in controlling the fraction of sample that enters the column. As a result, injection volumes are kept small, typically ranging from 1 microliter for capillary columns to 1-20 microliters for packed columns..
Scene 10 (6m 18s)
[Audio] The injector introduces a precise volume of sample into the gas chromatography system using a syringe or sampler, allowing for accurate control over the amount of sample injected. The injector also includes a purge valve that can be opened or closed to introduce the sample or purge residual gases from the system..
Scene 11 (6m 37s)
[Audio] The injector syringe is used to inject a sample into the gas chromatography system. This process involves opening the purge valve to remove any residual gases from the system, followed by closing the purge valve and using the injector syringe to inject the sample. The injector syringe can be filled with helium gas, denoted by the symbol "He", which carries the sample through the system..
Scene 12 (7m 0s)
[Audio] The goal of injecting a sample into a gas chromatography system is to deposit it into the column in the narrowest band possible. This is crucial because the shorter the band at the beginning of the chromatographic process, the more likely we are to obtain narrow peaks. These narrow peaks give us maximum resolution and sensitivity, which are essential for achieving precise and accurate results..
Scene 13 (7m 24s)
[Audio] The mechanism by which a portion of the injected solution is discarded, known as split injection, is used to handle concentrated samples, typically exceeding 0.1%. Only a small percentage of the sample, ranging from 1% to 5%, actually passes through the column during this process. This technique can be performed under isothermal conditions, allowing for fast injection speeds..
Scene 14 (7m 48s)
[Audio] Most of the sample, ranging from 85 to 100%, flows directly into the column during the splitless injection process, which is typically used for very dilute samples, usually less than 0.1%. This technique requires careful optimization, as it is controlled by a solenoid valve, and the injection speed is slower compared to other methods..
Scene 15 (8m 12s)
[Audio] The chromatographic column contains a stationary phase, many configurations of which depend on the specific needs of the analysis. The column can be seen as an image of a DeactiglasY glass column, which has been treated with silane for inertness and precision bore glass for reproducibility. Brass fittings and VespeI/graphite ferrules ensure a tight and leak-proof connection, while the column is available in both pre-packed and empty forms, providing flexibility for choosing the best option for a specific need..
Scene 16 (8m 43s)
[Audio] GC columns are the core components of any gas chromatography system. There are two primary types of GC columns: packed columns and capillary columns. Packed columns were used in early gas-liquid chromatography systems and are typically made of materials such as glass, Teflon, and aluminum. They have a length ranging from 2 to 3 meters and an inner diameter of approximately 3 millimeters. These columns are filled with a material known as a solid support, which retains the stationary phase essential for the separation process..
Scene 17 (9m 16s)
[Audio] The GC columns used in modern gas chromatography systems are primarily capillary columns. These columns are now made mostly of fused silica, which allows them to be bent into coils for easier handling. They have smaller inner diameters, ranging from 0.1 to 1 millimeter, and longer lengths, typically between 10 and 50 meters. Like packed columns, capillary columns use the same stationary phases, which are coated on the inside of the column. This design enables more efficient separations and higher resolution than traditional packed columns..
Scene 18 (9m 51s)
[Audio] The liquid stationary phase interacts with the mobile phase, which is helium flowing at a rate of one milliliter per minute. This combination enables analysis of samples within a concentration range of 0.1 to 5 millimeters..
Scene 19 (10m 6s)
[Audio] The characteristics of capillary columns used in gas chromatography systems include their length, internal diameter, liquid stationary phase film thickness, and polarity. These parameters can significantly impact the performance of the column and the quality of the analysis results. Longer columns may provide better separation of compounds, while thinner films may improve peak resolution. Non-polar columns are often used for analyzing hydrocarbons, whereas polar columns are more suitable for analyzing biological molecules. Understanding these characteristics is essential for selecting the appropriate column for a specific application and optimizing the analytical conditions..
Scene 20 (10m 46s)
[Audio] The choice of stationary phase plays a crucial role in determining the selectivity of a gas chromatography system. Hundreds of phases are available, making it essential to understand how they work together to achieve the desired separation. Many phases can provide the same separation results, but this does not mean they are interchangeable. Some phases may have different brand names, but their properties remain the same. For capillary columns, the process of selecting a stationary phase becomes much simpler. The fundamental principle is that "like dissolves like", meaning polar phases are suitable for polar components, while non-polar phases are better suited for non-polar components. By considering these factors, we can optimize our stationary phase selection to achieve the best possible results in our gas chromatography analysis..
Scene 21 (11m 34s)
[Audio] The internal diameter of a chromatographic column plays a crucial role in determining its performance. A smaller internal diameter, often referred to as ID, enables better resolution, particularly for early-eluting compounds. This results in a more precise separation between these compounds. Conversely, larger internal diameters can lead to shorter analysis times, but may compromise on the resolution of early-eluting compounds. This trade-off is vital to consider when choosing the appropriate column for analysis..
Scene 22 (12m 6s)
[Audio] The silanization process involves the reaction of dimethylchlorosilane with the solid support material, resulting in the formation of a siloxane bond. This bond creates active adsorption sites on the surface of the material, allowing it to interact with and retain analytes. The reaction can be represented by the following equations: Si – O – C – Cl + CH3OH → Si – O – C – OCH3 + CH3; Si – O – C – OCH3 + CH3 → Si – OH + CH3CH3; Cl – Si – Cl + HCl → Dimethylchlorosilane (DMCS). The resulting DMCS molecule forms a strong covalent bond with the solid support material, creating a stable and reactive site for adsorption. This process is commonly used in gas-solid chromatography to modify the surface properties of the solid support material, enhancing its ability to retain and separate analytes..
Scene 23 (13m 9s)
[Audio] The stationary phases in gas chromatography play a crucial role in separating mixtures of compounds. These phases possess specific properties that enable them to effectively interact with the solutes being analyzed. Low volatility, thermal stability, and chemical inertness are essential characteristics of a good stationary phase. Additionally, the solvent properties of the stationary phase should optimize the retention factor, k', and selectivity, α. When selecting a stationary phase, polarity is often considered. This is because the solute and stationary phase must have similar polarities for effective interaction. As the old adage goes, "like dissolves like." This fundamental principle guides our choice of stationary phase, ensuring optimal performance in gas chromatography systems..
Scene 24 (14m 0s)
[Audio] The different types of stationary phases used in gas chromatography include cycloparaffin, squalane, polydimethyl siloxane, poly(phenylmethyldimethyl) siloxane, polyethylene glycol, and poly(dicyanoalkyldimethyl) siloxane. These phases have varying polarities, ranging from non-polar to highly polar, and are commonly used for analyzing specific types of molecules. For example, cycloparaffin and squalane are used for general-purpose analysis of non-polar compounds, while polyethylene glycol is used for analyzing polar compounds like free acids and alcohols..
Scene 25 (14m 36s)
[Audio] The gas chromatography system's output shows a series of peaks corresponding to different compounds in the sample. Each peak corresponds to a specific retention time, which is determined by the interaction between the molecule and the stationary phase within the column. By analyzing these peaks, we can identify the presence and relative amounts of each component in the mixture. This information is essential for understanding the composition of the sample and making informed decisions about its properties and behavior..
Scene 26 (15m 6s)
[Audio] Gas-solid chromatography is based on the principle of adsorption, where gaseous substances interact with solid surfaces. This technique is commonly used to separate atmospheric gases, such as oxygen, nitrogen, argon, carbon dioxide, hydrogen sulfide, and carbon monoxide, among others. Both packed and open tubular columns can be used in this type of chromatography..
Scene 27 (15m 30s)
[Audio] Gas-Solid Chromatography is another type of chromatography where we use a solid surface to separate gaseous substances. This method is commonly used to separate atmospheric gases such as oxygen, nitrogen, argon, carbon dioxide, hydrogen sulfide, and carbon monoxide. We can perform this technique using both packed and open tubular columns..
Scene 28 (15m 55s)
[Audio] The gas chromatography system is designed to analyze halogenated pesticides by separating them from other substances using various components. Filters and traps remove impurities from the sample, and then air and hydrogen gas carriers transport the sample through the column, where it's separated based on its properties. The data system records the results, while detectors monitor the separation process. Regulators control the flow rates, and purge valves ensure the system remains clean. With this system, we can accurately identify and quantify halogenated pesticides..
Scene 29 (16m 29s)
[Audio] The characteristics of an ideal detector include sensitivity over a wide range, stability and reproducibility, linear response to analyte concentration, operation within a wide temperature range from ambient to 400 degrees Celsius, short response time to all solutes, similar response to all solutes, and non-destructive to the sample. These requirements ensure that the detector accurately measures the analyte's presence and concentration, providing reliable results..
Scene 30 (16m 57s)
[Audio] When an analyte elutes from the column, its thermal conductivity is reduced because most compounds have a lower thermal conductivity compared to that of helium or hydrogen, which are commonly used as carrier gases. This decrease in thermal conductivity is a characteristic feature of gas chromatography detectors..
Scene 31 (17m 16s)
[Audio] Temperature plays a crucial role in gas chromatography, as it can significantly impact the quality of the results. Initially, a low temperature of 45°C provides good resolution, but the process is too slow. Conversely, a higher temperature of 145°C speeds up the process, but compromises the resolution for early-eluting compounds. Typically, the best outcomes are achieved when the temperature is close to the boiling point of the analyte. When dealing with complex samples featuring a broad range of boiling points, temperature programming is necessary. This technique involves adjusting the temperature steps to optimize the results. For instance, a typical temperature program might involve gradual temperature increments over time, ensuring that each compound elutes under ideal conditions, ultimately leading to improved overall performance..
Scene 32 (18m 9s)
Typical Temperature Program. Time (min). 0. 60. 50C.
Scene 33 (18m 19s)
[Audio] Chemical derivatization is necessary because GC is best suited for separating volatile compounds that are thermally stable. However, this technique is not always applicable for compounds with high molecular weights or those containing polar functional groups. These compounds may not be sufficiently volatile, may tail badly, be too strongly attracted to the stationary phase, or even decompose during analysis. To overcome these limitations, chemical derivatization is performed prior to analysis. This process increases the volatility and decreases the polarity of compounds, reduces thermal degradation, increases detector response, and improves separation and reduces tailing. Derivatizing reagents can be classified into four main categories based on the type of reaction applied: silylation, acylation, alkylation, and esterification..
Scene 34 (19m 13s)
[Audio] In this final stage of our discussion, we are going to explore the different types of runs that can be performed using our gas chromatography system. One common type of run is programmable isothermal, where the temperature remains constant throughout the entire analysis. This is useful when analyzing samples that require a specific temperature range to achieve optimal separation. For example, some compounds may degrade quickly if exposed to high temperatures, so maintaining a constant temperature ensures their integrity during analysis. Another type of run is temperature programming, where the temperature starts at a low value and gradually ramps up to a higher value over time. This technique is often used when analyzing complex mixtures, as it allows for more efficient separation and detection of multiple components. By starting at a lower temperature, the system can focus on resolving the earliest-eluting peaks, and then gradually increasing the temperature to resolve later-eluting peaks. These two techniques demonstrate the flexibility and versatility of our gas chromatography system, allowing us to tailor our analyses to suit the specific needs of each sample. Whether we need to maintain a constant temperature or dynamically adjust the temperature to optimize separation, our system provides the tools to achieve accurate and reliable results..