Scene 1 (0s)
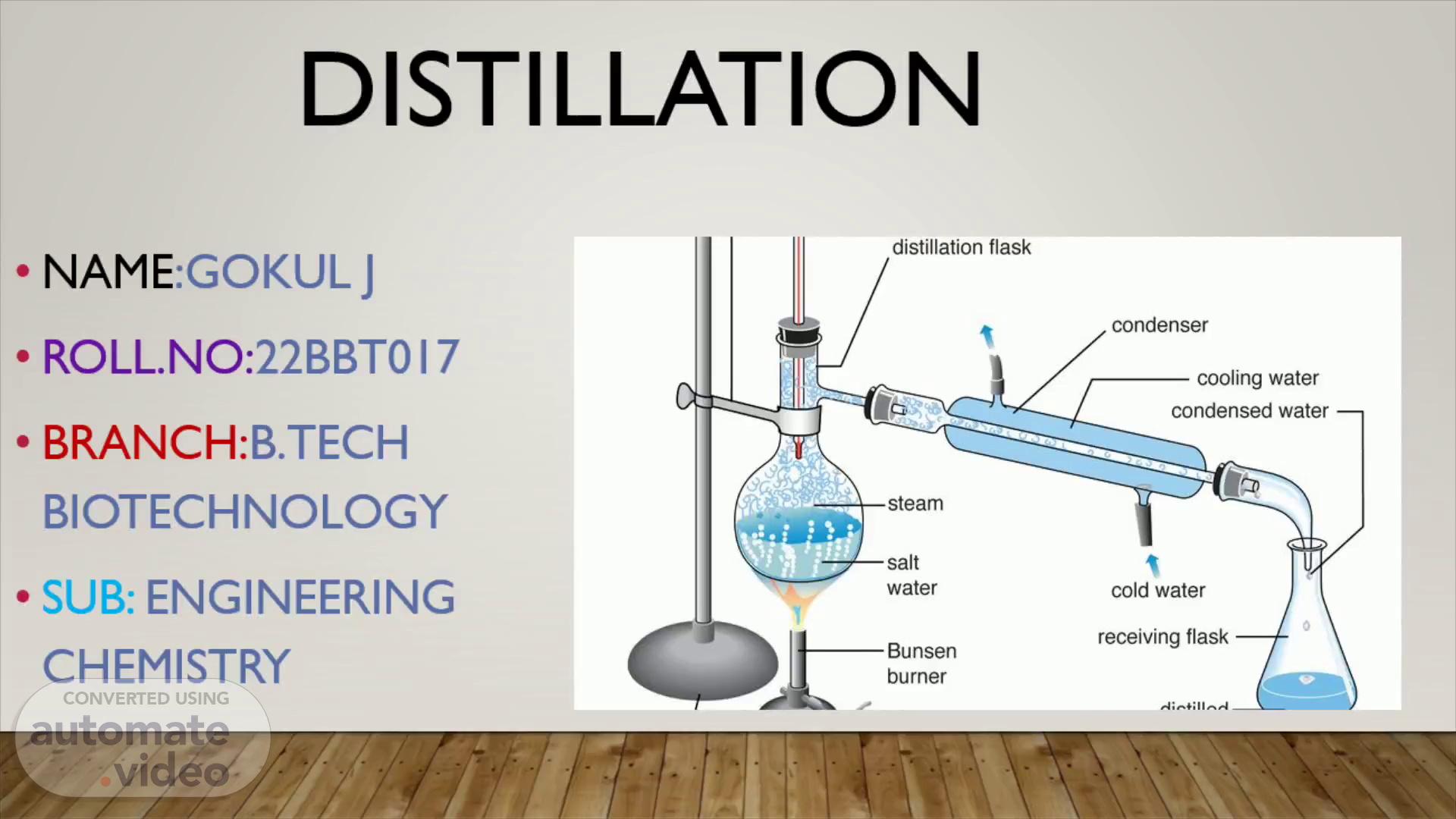
DISTILLATION. NAME :GOKUL J ROLL.NO: 22BBT017 BRANCH: B.TECH BIOTECHNOLOGY SUB: ENGINEERING CHEMISTRY.
Scene 2 (9s)
Definition Distillation is a process that involves separating and purifying liquids by heating them to create vapor and then cooling the vapor to produce concentrated liquid form..
Scene 3 (25s)
Types of distillation. FRACTIONAL DISTILLATION: Fractional distillation is a separation technique based on the differences in boiling points of the components in a liquid mixture. It uses a fractionating column to achieve separation by providing repeated vaporization and condensation cycles. The component with the lowest boiling point condenses and collects at the top, while higher boiling point components condense at lower levels within the column, resulting in the separation of the mixture. *Video in the next slide.
Scene 5 (53s)
TYPES OF distillation. STEAM DISTILLATION: Steam distillation separates volatile compounds from non-volatile substances effectively. it is gentle on heat-sensitive compounds, preserving their integrity during extraction. Steam distillation finds wide application in industries such as fragrance, flavor, herbal medicine, and chemical synthesis, offering a versatile method for obtaining pure and concentrated products. Here vapour pressure is extracted independently by an individual constituent on its own. The Vapour pressure of the system increases consequently, the two immiscible liquids start to boil when the vapour pressure of these liquids out place the atmospheric pressure. * Video in the next. Slide.