Scene 1 (0s)
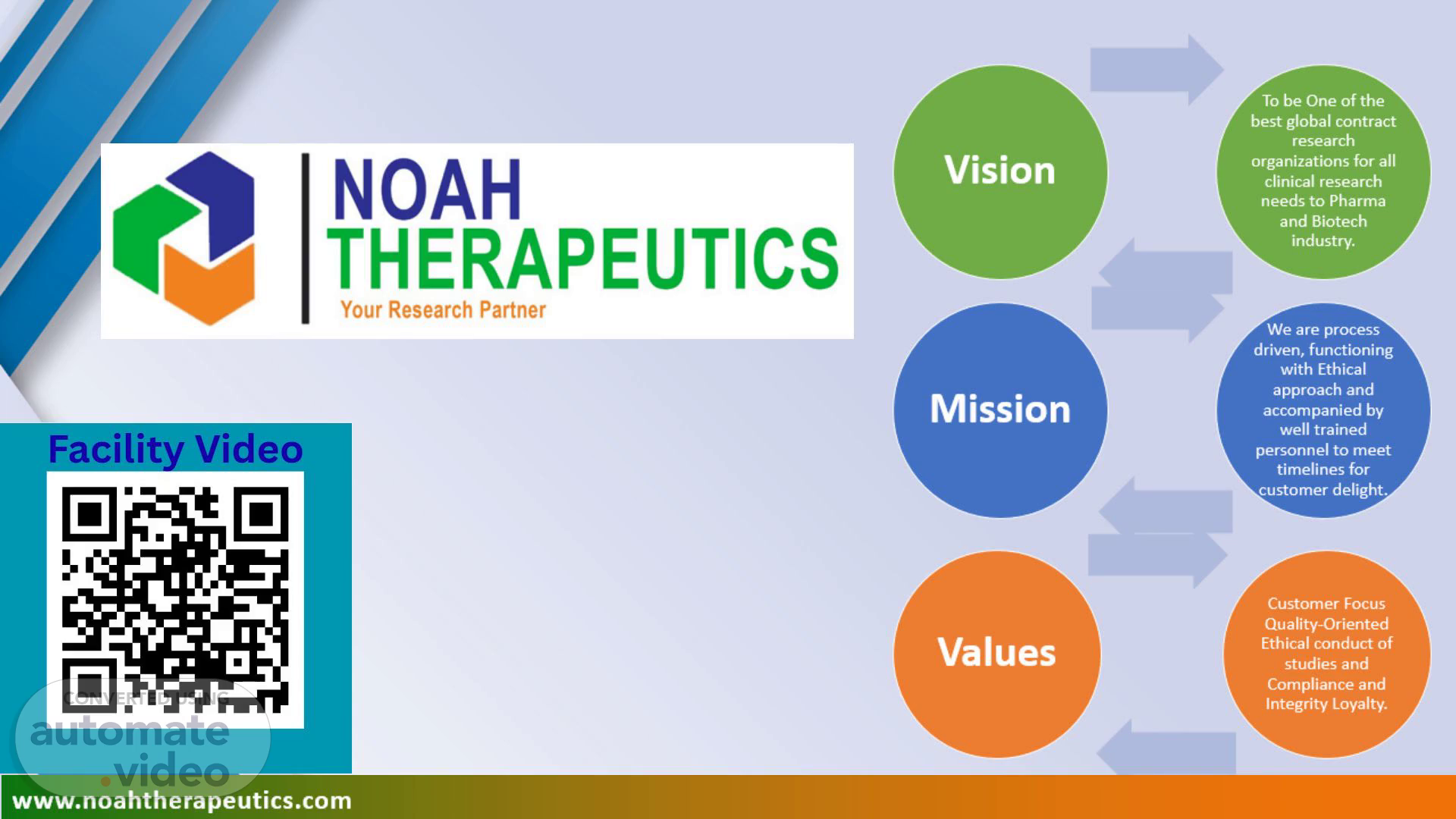
[Audio] Vision To be One of the best global contract research organizations for all clinical research needs to Pharma and Biotech industry. Mission We are process driven, functioning with Ethical approach and accompanied by well trained personnel to meet timelines for customer delight. Values Customer Focus Quality-Oriented Ethical conduct of studies and Compliance and Integrity Loyalty. www.noahtherapeutics.com.
Scene 2 (40s)
[Audio] Noah Therapeutics Introduction Incorporated in year 2022 and received approval from Central Drugs Standard Control Organization (CDSCO) in year 2023 Noah Therapeutics is 84 bedded, CRO based at Hyderabad, India offering end to end services for BA/BE, Patient PK studies and Clinical Trials (Phase I-IV). Noah Therapeutics is 21 CFR Part 11 & EU Annex 11 compliant with respect to computer and computerized systems Noah Therapeutics operates within a St. Theresa’s multispecialty hospital premises which provides it with unique advantages like access to varied patient population, management of medical emergencies, consultation with KOL’s from medical fraternity. St. Theresa’s hospital is a NABH accredited 300 Bedded Multi-Specialty teaching Hospital, with access to world class technologies and equipment like MRI, CT scan, Cardiac Cath-lab, State-of-the-art operation theaters, advanced laboratories and highly efficient emergency facilities including 30+ ultra modern ICU beds, Medical ICU, Surgical ICU which makes the hospital as one of the best in Hyderabad..
Scene 3 (2m 9s)
[Audio] Advantages of Noah Therapeutics Located within hospital premises ensuring volunteer safety in case of medical emergency Feasible for conducting Phase I and clinical trials by considering as site Easy access to subject matter clinicians for advisory roles, review and implementation of critical protocol design Key team members have more than 20 years of experience in conducting clinical studies Analytical Lab equipped with brand new 4500 LCMS/MS machine(s) Male & Female volunteer database, recruitment for special studies involving post menopausal females & geriatric population. The Clinical pharmacology unit meets global regulatory requirements and benchmarks - inspected and approved in past ( Pre-name change) by USFDA, WHO, EMA. www.noahtherapeutics.com.
Scene 4 (3m 12s)
[Audio] Management Dr P. Venkatesh. Founder and Managing Director, Clinician by background and secured Ph.D in the field of Clinical research post M.B.B.S. Has Over 2 decades of experience in conducting BA/BE and Clinical Phase Trial studies in healthy human and patients for various therapeutic areas and dosage forms. Complete understanding of best industry practices in line to regulatory requirements. Experience of conducting over 1000+ studies experience. Successfully handled multiple inspections from regulated regulatory bodies like US-FDA, EMEA, WHO, ANVISA, UK-MHRA, TGA,NPRA and others. N. Upendra Director N. Upendra has over 18 years of experience with excellent track record in the field of clinical research. He has extensive experience working on phases of clinical trials which includes Phase I,II,III and BA/BE Studies. He is well versed with the FDA regulations and ethical aspects of clinical trials. www.noahtherapeutics.com.
Scene 5 (4m 26s)
[Audio] Noah Therapeutics Credentials ANVISA awaiting for audit and GCC, NPRA application In-progress DCGI Approval Incorporated.
Scene 6 (4m 39s)
[Audio] Service Portfolio Medical Writing BA/BE studies Clinical Trials Bioanalytical Services Quality Assurance PK & Statistical Services Project Management BA/BE Key Departments Quality Assurance Medical Writing Clinical Department Project Management and supporting teams *Bio stats & Data Management Bioanalytical Research Total No of Employees : 86 Regular : 54 On call & Consultants: 24+03 Supporting staff: 05.
Scene 7 (5m 14s)
[Audio] Noah Therapeutics Services Bioavailability & Bioequivalence Phase-I Facility* Bioavailability/Bioequivalence (BA/BE) studies in mixed gender population, special populations. Pharmacokinetic (PK) and Pharmacodynamic (PD) studies Parenteral studies Dose Escalation studies Various Routes of Administration including patch studies, Inhalation studies , Topical etc. 12 Bedded Phase-I facility within *hospital premises for critical studies involving close monitoring of patients. Clinical Trials Phase II, III & IV clinical trials Extensive experience in Vaccines (1st In humans) Medical devices Nutraceutical Proof-of-concept studies Investigator-initiated trials Real World Evidence Studies Patient Pharmacokinetics (PK) studies Involving frequent sample collection , close monitoring in an hospital set-up across single/ multiple sites..
Scene 8 (6m 17s)
[Audio] Bioavailability & Bioequivalence Key Departments Clinical Facility Spread across 22000 square feet Access controlled 84 bedded capacity spread across Clinic-I with 54 beds and Clinic-II with 30 beds Synchronized clocks equipped with CCTV for continuous monitoring OVIS software for tracking central volunteers cross participation BEeDC software for internal registration of volunteers Pharmacy Equipped with storage space to handle on- going, retention and quarantine IP, Laminar Air Flow For Dispensing, Complete pharmacy has controlled temperature & monitoring system in place. Access control system, television equipped in volunteers waiting and housing area Informed consent area for registration and screening Separate cabins for obtaining informed consent with provision of AV recording Dedicated Sample Collection and Processing Area Dining and Recreation area Emergency nurse calling alert system Monochromatic light conditions are available Phase-I Facility* 12 bedded in Hospital Setup. www.noahtherapeutics.com.
Scene 9 (7m 32s)
[Audio] Bioavailability & Bioequivalence Key Departments Support Services: Medical Writing (Study synopsis, Protocol, IB, ICD, CRF writing, Literature search, Patient diaries Regulatory, IEC, IRB, SEC meetings Quality Control & Quality Assurance Data management PK & Statistical analysis Project Management Bioanalytical Facility Bioanalytical lab spreads over 4000 square feet area in separate building. Two LC-MS/MS Systems (SCIEX 4500) Audit trail and data security systems GLP and 21 CFR Part 11 & EU Annex 11 compliant Sample preparation laboratory equipped with centrifuges, SPE manifolds, refrigerators, vortex mixtures, pH meter, ultrasonic bath, Nitrogen evaporator and fume hoods with restricted access. Mettler Toledo Micro / Analytical balance. MD/MV of simple and complex drugs and metabolites as per regulatory requirements Deep Freezers Deep Freezers -70°C, [02] & Freezer -20°C, [01] Separate pharmaceutical refrigerators for storage of working/ reference standard and stock solutions Centralized Monitoring of Temperature Monochromatic light conditions are available.
Scene 10 (9m 10s)
[Audio] List of Developed Methods in Bioanalytical Amlodipine Apixaban Apremilast Atazanavir Axitinib Azilsartan Azithromycin Cabozantinib Canagliflozin Candesartan Carbamazepine Curcumin Dabigatran Dapagliflozin Dasatinib Dexlansoprazole Erlotinib Esomeprazole Flecainide Ibrutinib Lenalidomide Lenvatinib Linagliptin Mesalamine Metformin Mirabegron Naproxen Nitrofurantoin Omeprazole Pazopanib Raltegravir Rifaximin Ritonavir Rivaroxaban Rosuvastatin Tizanidine Tofacitinib Valsartan Curcumin metabolites- DMC, BDMC, THC www.noahtherapeutics.com.
Scene 11 (9m 49s)
[Audio] Clinical Trial Services. Clinical Trial Services.
Scene 12 (9m 58s)
[Audio] Team Experience in Clinical Trials First in Human Studies Experience Pneumococcal vaccine 15 valent COVID vaccine HPV vaccine IPV Vaccine Critical Studies / ICU Setup Studies Experience Cancer with metastatic stage Antibiotic-resistant infections Severe sepsis or septic shock TNK-TPA (Tenecteplase) Neurodegenerative diseases - Epilepsy Acute stroke Parkinson’s Pain Management Oncology Team Experience Imatinib Mesylate in patient-based population (CML) Nilotinib in patient-based population (CML) Trastuzumab in Breast Cancer Tislelizumab in Head & Neck, Lung Cancer Sunitinib in Renal Cell Carcinoma Rituximab- NSLC (Non Small Lung Cancer) Bevacizumab in Solid Tumour’s Denosumab – Bone Metastases Paclitaxel Protein bound Particles – Breast Cancer Trastuzumab – Breast cancer Cisplatin – Ovarian Cancer Carboplatin – Head and Neck Cancer Azacitidine – Myeloid Leukaemia Docetaxel – Prostate Cancer Neurology Team Experience Felbamate - Epilepsy Erenumab – Migraine Carbamazepine – Epilepsy Perampanel – Epilepsy TPA – Acute stroke Ticagrelol – TIA Cardiology Team Experience Inclisiran - Cholesterol metabolism Ramipril –Myocardial infraction Telmisartan + hydrochlorthiazide +amlodipine – Hypertension.
Scene 13 (12m 3s)
[Audio] Team Experience in Vaccine Clinical Trials S.No Indication Phase of the Trial 1. Meningococcal Conjugate Vaccine III 2. Covid-19 Vaccine mRna III 3. IPV Vaccine (Inactivated Polio Vaccine) I 4. TCV Vaccine (Typhoid Conjugate Vaccine) I 5. PCV (Pneumococcal Conjugate Vaccine) I 6. Intranasal Covid 19 vaccine I & II 7. Intradermal Covid 19 Vaccine I 8. Booster Dose Vaccine Covid 19 COVAXIN III 9. Booster Dose Vaccine Covid 19 mRna II & III 10. Corbevax Covid Vaccine I, II, III 11. Corbevax Covid Vaccine in Paediatrics II & III.
Scene 14 (13m 1s)
[Audio] Real‑World Evidence Studies, Nutraceuticals and Medical Devices Nutraceutical Studies Ongoing/Planned: Wound healing CKD Bioavailability study of antioxidant Iron Deficiency Haemorrhoids Pregnancy Vitamins & Minerals Fitness Supplements Real‑World Evidence Studies Team Experience: Two Diabetic Real‑world evidence studies Real-world observational study to evaluate dyslipidaemia Ultrasound AI device (Planning) Medical Devices Studies conducted: Single-Centre, Single Arm Study to evaluate the potential allergies to wound dressing by conducting Skin Prick Test in healthy adult human subjects (25 subjects). Human Repeat Insult Patch Test (HRIPT) to assess the cutaneous irritation and sensitization potential of wound dressing in healthy adult human subjects. (60 subjects) www.noahtherapeutics.com.
Scene 15 (14m 5s)
[Audio] Therapeutic Expertise Nephrology Neurology Nutraceuticals Oncology Ophthalmology Orphan / Rare Disease Psychiatry Pulmonology Rheumatology Cardiology Dermatology Endocrinology Gastroenterology Genetics Gynecology Health Wearables Infectious Disease Infertility.
Scene 16 (14m 30s)
[Audio] Clinical Facility Intensive Care Unit (ICU)-1 Clinical Area Intensive Care Unit (ICU)-2 www.noahtherapeutics.com.
Scene 17 (14m 43s)
[Audio] Sample Collection Area Laminar Air Flow Pharmacy Sample Separation Room Archival Room -70°Freezer Sample Processing Area.
Scene 18 (14m 54s)
[Audio] Bioanalytical Facility LC-MS/MS. Bioanalytical Facility.
Scene 19 (15m 2s)
[Audio] Deep Freezers (-70°C &-20°C) Fume Hood Pharmaceutical refrigerators for storage of working/ reference standard and stock solutions Micro &Analytical Balances Mettler Toledo www.noahtherapeutics.com.
Scene 20 (15m 23s)
[Audio] Corporate Office www.noahtherapeutics.com website: www.noahtherapeutics.com.
Scene 21 (15m 35s)
[Audio] Corporate Office Helsinki Hall Belmont Hall.
Scene 22 (15m 46s)
[Audio] Thank You Contact Details: Dr. P. Venkatesh, Managing Director, M:+91 9440383778 e-mail: [email protected] Arvind Koul, Head- BD, M:+91 9740799778 e-mail:[email protected] Address: Noah Therapeutics Pvt. Ltd, D.No.7-1-645/A, Erragadda, Sanath Nagar, Hyderabad, Telangana 500018, India. Website: www.noahtherapeutics.com.