Scene 1 (0s)
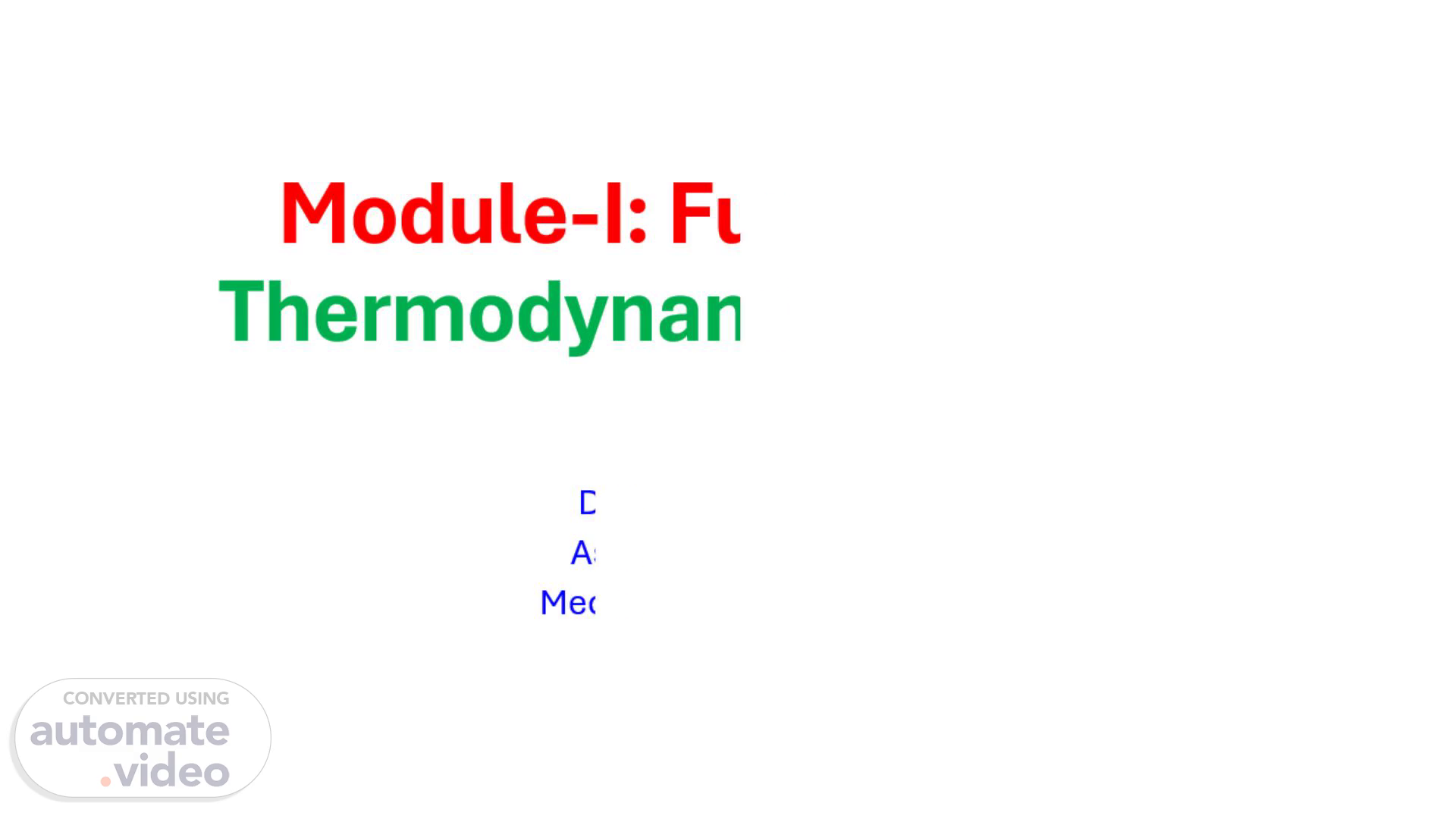
Module-I: Fundamental Thermodynamic Concepts. By Dr. B. Sambi Reddy Associate Professor Mechanical Engineering.
Scene 2 (8s)
Content:. Properties, Process, State, and Cycle: Intensive & extensive properties. Thermodynamic process and state point. Thermodynamic cycle with examples (power & refrigeration cycle)..
Scene 3 (20s)
What is a Thermodynamic State?. A state refers to the condition of a system at a specific moment in time. If even a single property of a system changes, its state changes. For a system to be in a definite state, it must be in thermodynamic equilibrium, meaning there are no unbalanced potentials (e.g., thermal, mechanical, or chemical)..
Scene 4 (38s)
What are Thermodynamic Properties?. A measurable characteristics of a system. Properties are the macroscopic, observable characteristics of a system. They describe the system's state and can be measured or calculated. Examples: Pressure (P), Temperature (T), Volume (V), Density (ρ), and Internal Energy (U). Properties are point functions, meaning their value depends only on the state of the system, not on how the system arrived at that state..
Scene 5 (59s)
THERMODYNABIIC PROPERTIES INTENSIVE PROPERTY EXTENSIVE PROPERTY.
Scene 6 (1m 4s)
Extensive Properties. Definition: Properties that depend on the amount of matter or the size of the system. Key Characteristic: They are additive. If you combine two systems, the value of the extensive property for the combined system is the sum of the individual values. Examples: Mass (m): The total mass of the system. Volume (V): The total space occupied by the system. Total Energy (E): The sum of all forms of energy in the system. Total Entropy (S): A measure of the total disorder of the system..
Scene 7 (1m 30s)
Intensive Properties:. Definition: Properties that are independent of the amount of matter or the size of the system. Key Characteristic: They are not additive. If you divide a system into parts, each part will have the same value of the intensive property as the original system. Examples: Pressure (P): The force per unit area. Temperature (T): The measure of hotness or coldness. Density (ρ): Mass per unit volume (ρ=m/V). Specific Volume (v): Volume per unit mass (v=V/m)..
Scene 8 (1m 55s)
Intensive Properties Temperature Extensive Properties Boiling Point Volume Color Mass Luster Size Weight Hardness Length.
Scene 9 (2m 1s)
What is a Thermodynamic Process?. A process is the path or change of state that a system undergoes as it moves from an initial state to a final state. The path of a process is the series of intermediate states the system passes through. Processes are defined by the properties that remain constant: Isochoric Process: Constant volume (V). Isobaric Process: Constant pressure (P). Isothermal Process: Constant temperature (T). Isentropic Process: Constant entropy (s)..
Scene 10 (2m 22s)
Summary Table. Types of Properties Mass Example Type of Function Intensive Properties Independent P, T, ρ, v Point Function Extensive Properties Dependent m, V, E, S Point Function.
Scene 11 (2m 35s)
What is a Thermodynamic Cycle?. A cycle is a sequence of processes that begins and ends at the same initial state. In a cycle, the system returns to its original condition. Since properties are point functions, the net change in all properties (e.g., ΔP, ΔT, ΔV) for a complete cycle is zero. Cycles are fundamental to the operation of heat engines and refrigeration systems..
Scene 12 (2m 54s)
[image] Thermodynamic Cycles GeeksforGeeks.
Scene 13 (3m 2s)
Thank you.