Scene 1 (0s)
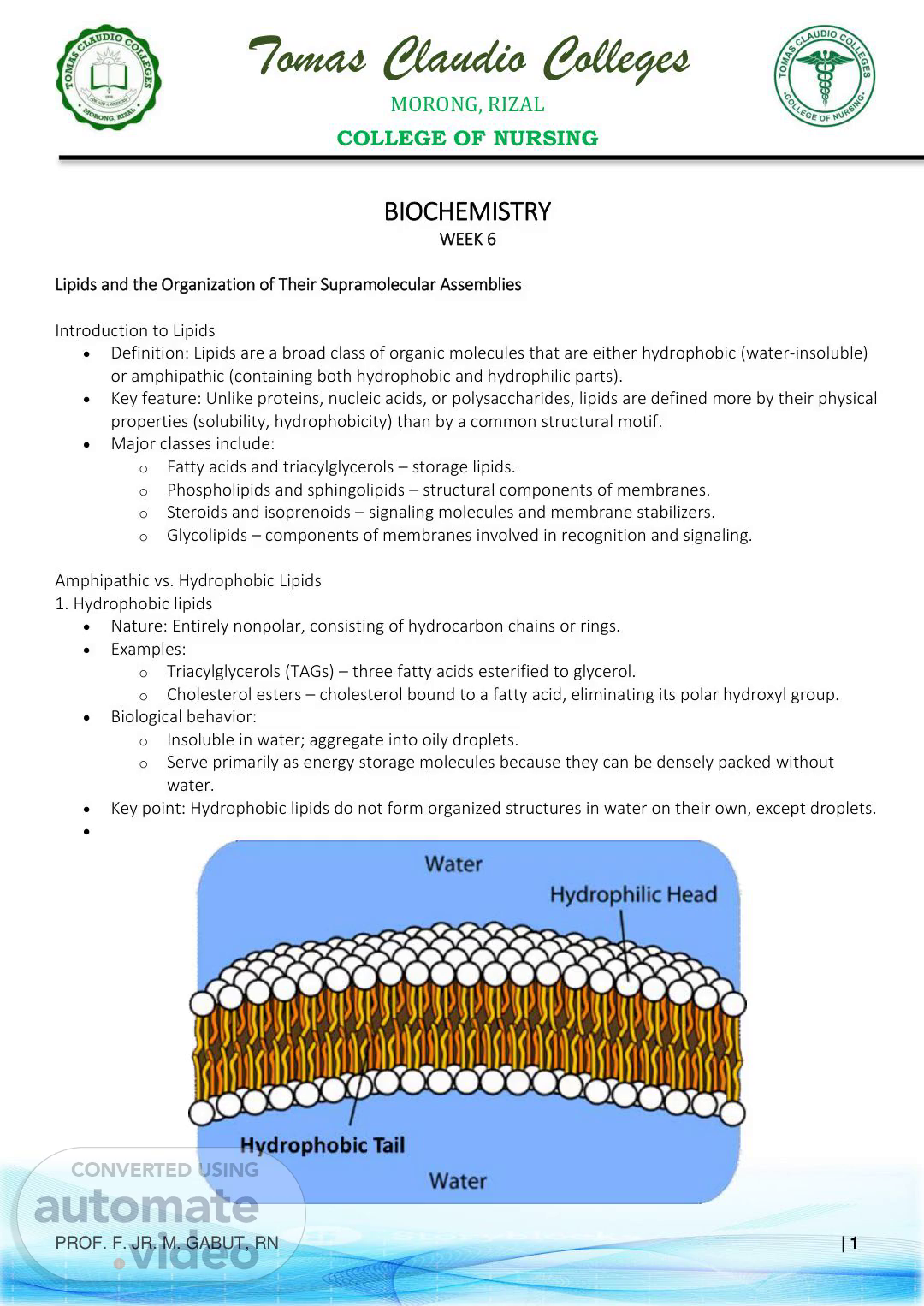
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 1 BIOCHEMISTRY WEEK 6 Lipids and the Organization of Their Supramolecular Assemblies Introduction to Lipids • Definition: Lipids are a broad class of organic molecules that are either hydrophobic (water-insoluble) or amphipathic (containing both hydrophobic and hydrophilic parts). • Key feature: Unlike proteins, nucleic acids, or polysaccharides, lipids are defined more by their physical properties (solubility, hydrophobicity) than by a common structural motif. • Major classes include: o Fatty acids and triacylglycerols – storage lipids. o Phospholipids and sphingolipids – structural components of membranes. o Steroids and isoprenoids – signaling molecules and membrane stabilizers. o Glycolipids – components of membranes involved in recognition and signaling. Amphipathic vs. Hydrophobic Lipids 1. Hydrophobic lipids • Nature: Entirely nonpolar, consisting of hydrocarbon chains or rings. • Examples: o Triacylglycerols (TAGs) – three fatty acids esterified to glycerol. o Cholesterol esters – cholesterol bound to a fatty acid, eliminating its polar hydroxyl group. • Biological behavior: o Insoluble in water; aggregate into oily droplets. o Serve primarily as energy storage molecules because they can be densely packed without water. • Key point: Hydrophobic lipids do not form organized structures in water on their own, except droplets. •.
Scene 2 (49s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 2 2. Amphipathic lipids • Nature: Contain polar (hydrophilic) head groups and nonpolar (hydrophobic) tails. • Examples: o Phospholipids (e.g., phosphatidylcholine, phosphatidylserine) – major membrane lipids. o Glycolipids – lipids with carbohydrate head groups involved in cell recognition. o Cholesterol (unesterified) – weakly amphipathic because of its hydroxyl group. • Biological behavior: o Self-assemble in water to minimize unfavorable interactions between hydrophobic tails and polar solvent. o Form micelles, bilayers, and vesicles, depending on their geometry. o Provide the structural framework of biological membranes. Physical basis for amphipathic behavior • The hydrophobic effect: Water molecules form ordered cages (clathrate structures) around nonpolar groups → this is entropically unfavorable. When hydrophobic groups cluster together, fewer water molecules are ordered, increasing entropy and stabilizing the assembly. • Result: Amphipathic molecules spontaneously organize into supramolecular structures with minimal free energy. Biological Roles of Lipids 1. Energy storage • Triacylglycerols (TAGs) are the main long-term energy reservoir in animals. o Highly reduced carbon atoms → yield more ATP upon oxidation than carbohydrates. o Anhydrous nature: Lipid droplets do not bind water → compact energy storage (about 2× energy density of glycogen). • Metabolic importance: During fasting or extended exercise, fatty acids from TAGs are mobilized via lipolysis and oxidized to produce ATP. • Clinical note: Excess TAG storage leads to obesity; insufficient storage occurs in lipodystrophy..
Scene 3 (1m 46s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 3 2. Membrane structure • Phospholipids and sphingolipids form the bilayer framework of cell membranes and organelles. • Membrane properties provided by lipids: o Barrier function: Selectively permeable to ions and molecules. o Fluidity and flexibility: Controlled by lipid composition (e.g., unsaturated fatty acids increase fluidity; cholesterol modulates rigidity). o Asymmetry: Inner and outer leaflets have different lipid compositions important for signaling (e.g., exposure of phosphatidylserine in apoptosis). • Dynamic nature: Membranes are not static—lipids diffuse laterally and rearrange, supporting cell signaling and transport..
Scene 4 (2m 15s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 4 3. Signaling roles • Steroid hormones (derived from cholesterol): regulate development, metabolism, reproduction (e.g., cortisol, estrogen, testosterone). • Eicosanoids (derived from arachidonic acid): local mediators in inflammation, immunity, and blood clotting (e.g., prostaglandins, leukotrienes, thromboxanes)..
Scene 5 (2m 35s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 5 • Intracellular second messengers: o Diacylglycerol (DAG) and phosphoinositides activate protein kinase C and regulate calcium signaling. o Sphingosine-1-phosphate (S1P) regulates cell proliferation and migration. • Clinical relevance: o Nonsteroidal anti-inflammatory drugs (NSAIDs, e.g., aspirin) block prostaglandin synthesis by inhibiting cyclooxygenase (COX). o Statins lower cholesterol synthesis, reducing substrate for steroid hormone and bile acid production but improving cardiovascular health. Self-Assembly of Lipids in Aqueous Media.
Scene 6 (3m 0s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 6 1. Introduction • Lipids do not float randomly in water; their chemical nature determines how they organize. • Hydrophobic vs. amphipathic behavior: o Purely hydrophobic lipids (like triacylglycerols) aggregate into oily droplets. o Amphipathic lipids (like phospholipids, glycolipids, and unesterified cholesterol) self-organize to minimize contact between hydrophobic regions and water. • This self-organization is spontaneous and thermodynamically driven, producing stable supramolecular structures critical for cell membranes, transport, and drug delivery. 2. Hydrophobic Effect as the Driving Force Basic thermodynamics • Water is highly ordered due to extensive hydrogen bonding. • When nonpolar molecules are exposed to water: o Water molecules arrange into ordered cages (clathrates) around them, decreasing entropy (ΔS < 0). o This is energetically unfavorable because the system loses randomness. • When hydrophobic regions cluster together: o Fewer water molecules are forced into an ordered structure. o Entropy increases (ΔS > 0), making the process spontaneous (ΔG < 0). 3. Supramolecular Structures Formed by Amphipathic Lipids A. Micelles.
Scene 7 (3m 44s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 7 • Shape: Spherical aggregates with hydrophobic tails inward and polar head groups outward, facing water. • Lipid requirement: Single-tailed amphipathic lipids with a conical shape (e.g., detergents, bile salts, fatty acids). • Critical micelle concentration (CMC): The threshold concentration at which micelles spontaneously form. • Function: o Solubilize hydrophobic molecules in an aqueous environment. o Facilitate lipid digestion and absorption (bile salts form micelles to emulsify dietary fats). B. Bilayers • Shape: Planar double-layered sheets with hydrophobic tails facing inward and hydrophilic heads outward. • Lipid requirement: Double-tailed cylindrical amphipathic lipids (e.g., phosphatidylcholine, sphingomyelin). • Function: o Structural basis of all biological membranes. o Provide a semi-permeable barrier to ions and polar molecules. C. Vesicles (Liposomes) • Shape: Closed, spherical bilayers surrounding an internal aqueous compartment. • Formation: When lipid bilayers curve and seal to avoid exposing hydrophobic edges. • Function: o Compartmentalization of biochemical reactions. o Experimental and therapeutic use as drug delivery systems (liposomal formulations improve solubility, reduce toxicity, and allow targeted delivery). 5. Biological and Practical Examples Micelle Formation by Detergents • Bile salts in the small intestine emulsify dietary fats → micelles make TAGs accessible to pancreatic lipases..
Scene 8 (4m 38s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 8 • Synthetic detergents (e.g., SDS) are used in laboratories to solubilize hydrophobic proteins or lipids. Liposome Drug Delivery Systems • Liposomal drugs (e.g., liposomal doxorubicin) encapsulate hydrophilic drugs in aqueous cores or hydrophobic drugs in the bilayer. • Advantages: o Improved solubility for poorly soluble drugs. o Reduced systemic toxicity by controlled release. o Targeted delivery by attaching antibodies or ligands to the liposome surface. • Clinical impact: Used in cancer therapy, antifungal therapy (liposomal amphotericin B), and vaccine delivery. The Structure of Biological Membranes 1. Introduction to Biological Membranes • All cells are bounded by a plasma membrane, and eukaryotic cells also contain internal membranes that form organelles (nucleus, ER, mitochondria, etc.). • Functions of membranes: o Barrier function – separate cell contents from the external environment. o Compartmentalization – allow organelles to maintain specialized environments. o Communication – receptors in membranes receive and transmit signals. o Transport – regulate movement of ions and molecules. o Energy conversion – mitochondrial and chloroplast membranes perform oxidative phosphorylation and photosynthesis. 2. The Fluid Mosaic Model (Singer and Nicolson, 1972) Key principles • Membranes are dynamic, not rigid: lipids and proteins are free to move laterally within the plane of the membrane, giving it “fluid” character. • Mosaic nature: composed of a heterogeneous mixture of lipids, proteins, and carbohydrates arranged in a discontinuous pattern..
Scene 9 (5m 36s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 9 • Asymmetry: the lipid and protein composition of the inner leaflet differs from the outer leaflet, giving functional polarity. Structural organization • Lipid bilayer as the basic structural scaffold. • Proteins interspersed like “tiles in a mosaic,” performing transport, signaling, and enzymatic roles. • Carbohydrates attached to lipids (glycolipids) or proteins (glycoproteins) on the extracellular surface for cell recognition. 3. Lateral Mobility of Membrane Components Lipid dynamics • Lateral diffusion: phospholipids move side-to-side within the same leaflet very rapidly (microseconds to milliseconds). • Rotation and flexion: individual lipid molecules rotate around their long axis and flex hydrocarbon tails. • Flip-flop: movement from one leaflet to the other is very slow without enzymes (flipases, flopases, scramblases)..
Scene 10 (6m 12s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 10 Protein dynamics • Integral membrane proteins also diffuse laterally, but more slowly than lipids due to their size and interactions with cytoskeleton or extracellular matrix. • Experimental evidence: Fluorescence Recovery After Photobleaching (FRAP) shows that when a membrane patch is photobleached, fluorescence recovers as unbleached molecules diffuse in. Physiological importance • Membrane fluidity is crucial for: o Transport protein function. o Signal transduction. o Endocytosis, exocytosis, and vesicle fusion. • Regulation of fluidity: o Fatty acid saturation: unsaturated fatty acids increase fluidity; saturated fatty acids make membranes more rigid. o Cholesterol content: acts as a “fluidity buffer,” preventing membranes from becoming too rigid at low temperatures or too fluid at high temperatures. 4. Integral vs. Peripheral Membrane Proteins Integral (intrinsic) proteins • Embedded deeply in the lipid bilayer. • Often have hydrophobic transmembrane α-helices or β-barrels. • Functions: o Ion channels, transporters, pumps. o Signal receptors (e.g., GPCRs). o Cell adhesion molecules. Peripheral (extrinsic) proteins • Loosely associated with the membrane surface through electrostatic interactions or binding to integral proteins. • Functions: o Cytoskeletal anchoring. o Signal transduction. o Enzymatic regulation. Carbohydrate components.
Scene 11 (7m 2s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 11 • Glycolipids and glycoproteins are exclusively on the outer leaflet, forming the glycocalyx, which mediates cell-cell recognition and protection. 5. Asymmetry of Lipid Distribution (Inner vs. Outer Leaflet) Outer leaflet lipids • Rich in phosphatidylcholine and sphingomyelin. • Display glycolipids and glycoproteins → carbohydrate chains project outward for cell recognition. Inner leaflet lipids • Rich in phosphatidylserine (PS), phosphatidylethanolamine (PE), and phosphatidylinositol (PI). • These play roles in intracellular signaling and anchoring proteins to membranes. Physiological and pathological roles • Phosphatidylserine externalization: normally restricted to the inner leaflet; its exposure on the cell surface marks cells for apoptosis, allowing macrophages to recognize and remove dying cells. • Signal transduction: PI is phosphorylated to PIP2 and PIP3, key messengers for intracellular signaling pathways..
Scene 12 (7m 39s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 12.
Scene 13 (7m 49s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 13 6. Clinical Link: Membrane Defects in Hereditary Spherocytosis Overview • Hereditary spherocytosis (HS): a genetic defect affecting red blood cell (RBC) membrane proteins (e.g., spectrin, ankyrin, band 3). • Leads to loss of membrane structural integrity, making RBCs spherical instead of biconcave. Consequences • Spherical RBCs are less deformable, causing premature destruction (hemolysis) in the spleen. • Symptoms: anemia, jaundice, splenomegaly. • Laboratory findings: increased osmotic fragility test, spherocytes on peripheral smear. Membrane biology relevance • Shows how defects in lipid–protein interactions can compromise cell survival. • Demonstrates that membranes are supported by an underlying cytoskeleton, not just a lipid bilayer. Treatment.
Scene 14 (8m 23s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 14 • Splenectomy is often performed to prevent destruction of RBCs, though the membrane defect remains..
Scene 15 (8m 36s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 15 The Structure of Lipoproteins 1) Lipoprotein architecture: how to float fat through water Problem: Neutral lipids (triacylglycerols, cholesteryl esters) are hydrophobic. Solution: Pack them into spherical nanoparticles with a detergent-like coat. • Hydrophobic core: o Triacylglycerols (TAG) o Cholesteryl esters (CE) • Amphipathic surface monolayer: o Phospholipids (polar heads face plasma; acyl tails face core) o Unesterified (free) cholesterol o Apolipoproteins (apos) — the “IDs and control panels”: ▪ ApoB-48 (chylomicrons, intestine origin): assembly & structural scaffold. ▪ ApoB-100 (VLDL/IDL/LDL, liver origin): structural; ligand for LDL receptor..
Scene 16 (9m 5s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 16 ▪ ApoA-I (HDL): structural; activates LCAT; ligand for SR-BI. ▪ ApoC-II: activates lipoprotein lipase (LPL)—key for TAG clearance. ▪ ApoC-III: inhibits LPL and hepatic uptake (↑TG when high). ▪ ApoE: ligand for remnant receptors (LRP1/LDLR) → hepatic clearance..
Scene 17 (9m 24s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 17.
Scene 18 (9m 34s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 18 2) Classes & functions: size ↔ density ↔ function Rule of thumb: Bigger → TAG-rich → lower density. Smaller → cholesterol-rich → higher density. Class Size (largest → smallest) Core cargo (major) Signature apos Primary function / route Chylomicron Very large Dietary TAG B-48, A-I, C- II, E Deliver intestinal TAG to tissues (LPL) → liver takes remnants (ApoE) VLDL Large Hepatic TAG B-100, C-II, E Deliver liver TAG to tissues (LPL) → becomes IDL IDL Medium TAG + CE (mixed) B-100, E Half cleared by liver (ApoE), half remodeled to LDL LDL Smaller Cholesteryl ester B-100 Deliver cholesterol to tissues via LDL receptor (LDLR) HDL Smallest (dense) CE (after LCAT) A-I (±A-II) Reverse cholesterol transport → liver via SR-BI; exchange via CETP.
Scene 19 (10m 10s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 19 3) Metabolic pathways (the “traffic map”) A) Exogenous pathway (dietary fat → body) 1. Intestine packages dietary TAG + cholesterol into nascent chylomicrons (ApoB-48). 2. In plasma, they pick up ApoC-II (for LPL) and ApoE from HDL. 3. LPL (anchored on capillary endothelium; adipose, heart, muscle) hydrolyzes TAG → fatty acids (stored or oxidized) + glycerol. 4. Chylomicron remnants (cholesterol-rich, ApoE) are taken up by the liver via LRP1/LDLR. B) Endogenous pathway (liver fat → body) 1. Liver secretes VLDL (ApoB-100) carrying hepatic TAG. 2. LPL trims VLDL → IDL (TAG↓, CE↑). 3. IDL: ~50% is cleared by liver (ApoE-dependent); remainder loses more TAG (hepatic lipase) → LDL. 4. LDL delivers CE to cells via LDL receptor (binds ApoB-100); most LDL is taken up by the liver..
Scene 20 (10m 49s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 20 C) Reverse cholesterol transport (periphery → liver) 1. Nascent HDL (discoidal; ApoA-I) is secreted by liver & intestine. 2. Cells export free cholesterol to HDL via ABCA1/ABCG1 → LCAT (activated by ApoA-I) esterifies cholesterol → moves into the HDL core (matures HDL). 3. HDL delivers CE to liver directly via SR-BI and/or exchanges CE for TAG with apoB particles via CETP (then apoB particles bring CE to liver). Enzymes & players to remember: • LPL: clears TAG from chylomicrons/VLDL (needs ApoC-II). • LCAT: esterifies cholesterol on HDL (needs ApoA-I). • Hepatic lipase: remodels IDL→LDL and HDL size. • CETP: swaps HDL-CE for VLDL/LDL-TAG. • LDLR/LRP1/SR-BI: key hepatic receptors for clearance. 4) “Bad” LDL vs “Good” HDL — what that really means • LDL (“bad”): Main courier of cholesterol to tissues; when excessive, it can deposit cholesterol in the arterial wall—especially when retained and oxidatively modified. • HDL (“good”): Returns cholesterol from tissues to liver and supports anti-inflammatory, antioxidant functions (via ApoA-I, enzymes). • Nuance: High HDL-C isn’t always protective if HDL is dysfunctional; ApoB (particle number) can be a better risk marker than LDL-C alone. 5) Pathophysiology: how oxidized LDL (oxLDL) drives atherosclerosis 1. Subendothelial retention: ApoB-containing particles (LDL, remnants) bind arterial proteoglycans and linger. 2. Oxidative modification: Reactive oxygen species, myeloperoxidase, lipoxygenases oxidize LDL lipids & ApoB-100 → oxLDL. 3. Scavenger receptor uptake: Macrophages take up oxLDL via SR-A, CD36 (not down-regulated by intracellular cholesterol) → foam cells. 4. Fatty streak → plaque: Foam cells release cytokines, recruit smooth muscle, lay extracellular matrix → fibrous cap over lipid core. 5. Instability & events: Thin caps can rupture → thrombosis → MI/stroke. Risk amplifiers: diabetes/insulin resistance (small dense LDL, high remnants), hypertension, smoking, chronic inflammation, high Lp(a). 6) Therapeutic targets & what they change on the map • Statins (↓HMG-CoA reductase) → ↑LDLR expression → ↓LDL-C/apoB. • Ezetimibe (↓NPC1L1 intestinal cholesterol absorption) → additional LDL-C lowering. • PCSK9 inhibitors (monoclonal antibodies or siRNA) → preserve LDLR → major LDL-C/apoB lowering. • Bempedoic acid (↓ATP-citrate lyase) → upstream statin-like effect, LDL-C↓. • Bile acid sequestrants → ↑bile acid excretion → LDL-C↓ (watch TG). • Fibrates (PPAR-α) → ↑LPL, ↓ApoC-III → TG↓, ↑HDL-C (variable). • Omega-3 fatty acids (EPA/DHA) → TG↓ in severe hypertriglyceridemia. • Niacin (less used) → ↓VLDL synthesis, ↑HDL-C; limited by side effects. • Lifestyle (dietary pattern, weight loss, physical activity) → improves TG, HDL-C, LDL particle quality..
Scene 21 (11m 54s)
Tomas Claudio Colleges MORONG, RIZAL COLLEGE OF NURSING PROF. F. JR. M. GABUT, RN | 21 7) Clinical pearls & inherited disorders • Non-HDL-C (= TC – HDL-C) tracks all apoB particles; ApoB directly counts atherogenic particle number. • Triglycerides ≥500 mg/dL → pancreatitis risk; first goal is TG lowering. • Familial hypercholesterolemia (FH) (LDLR/ApoB/PCSK9 variants): very high LDL-C, tendon xanthomas, early ASCVD. • Familial dysbetalipoproteinemia (ApoE2/E2): remnant accumulation, palmar xanthomas, high TC & TG. • LPL or ApoC-II deficiency: severe chylomicronemia, eruptive xanthomas, pancreatitis. • Abetalipoproteinemia (MTTP deficiency): no ApoB lipoproteins → fat malabsorption, neurologic issues. • Tangier disease (ABCA1 defect): very low HDL, orange tonsils, neuropathy..