Scene 1 (0s)
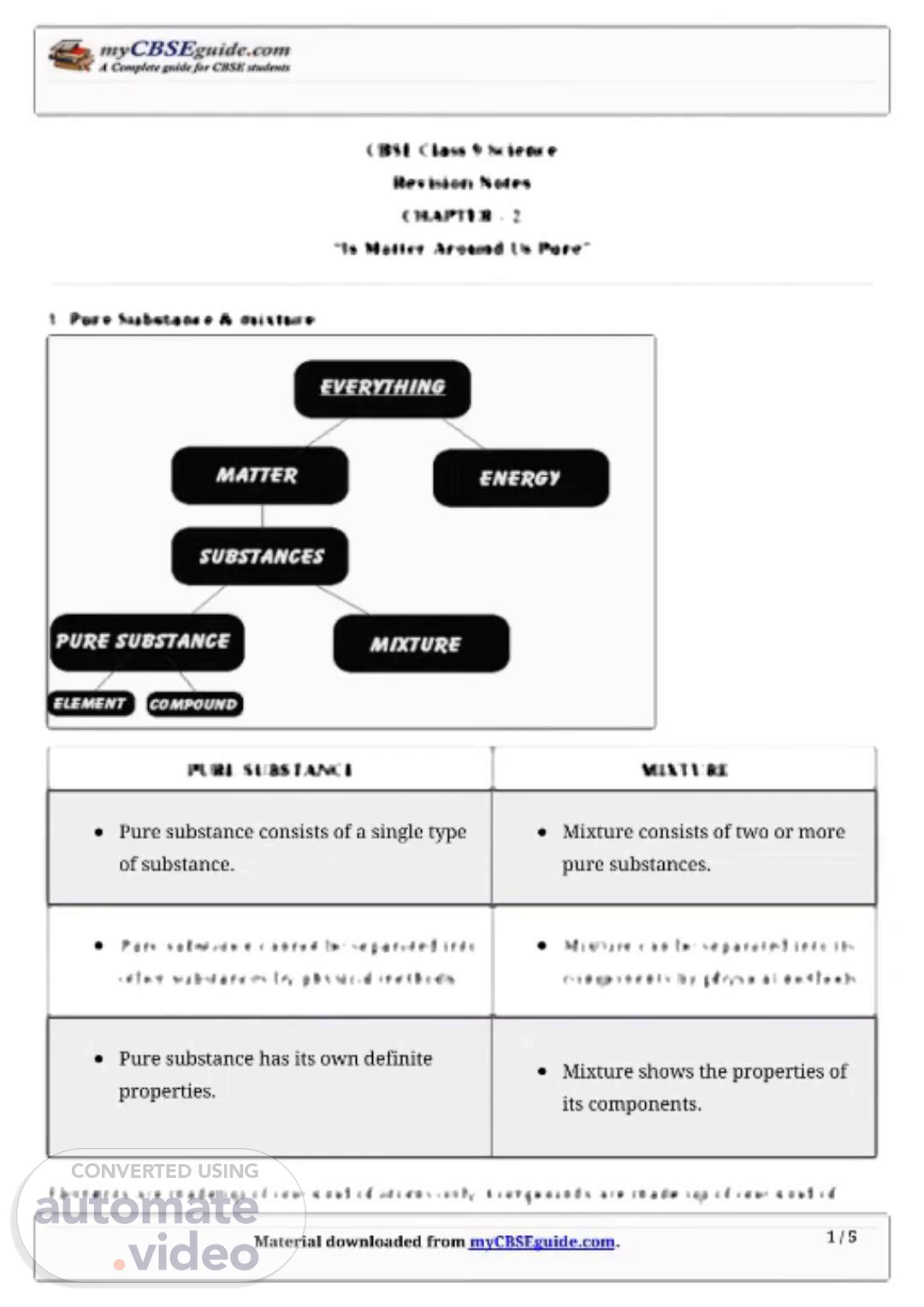
Material�downloaded�from�myCBSEguide.com. 1�/�5 CBSE�Class�9�Science Revision�Notes CHAPTER�–�2 “Is�Matter�Around�Us�Pure” 1.�Pure�Substance�&�mixture PURE�SUBSTANCE MIXTURE Pure�substance�consists�of�a�single�type of�substance. Mixture�consists�of�two�or�more pure�substances. Pure�substance�cannot�be�separated�into other�substances�by�physical�methods. Mixture�can�be�separated�into�its components�by�physical�methods. Pure�substance�has�its�own�definite properties. Mixture�shows�the�properties�of its�components. Elements�are�made�up�of�one�kind�of�atoms�only.�Compounds�are�made�up�of�one�kind�of.
Scene 2 (1m 53s)
[Audio] In our previous slide, we learned about the concepts of pure substances and mixtures. Now, we will explore the various types of mixtures. Mixtures can be grouped according to their physical states - solid, liquid, and gas. For example, salt and sugar are solid in their pure form, but when mixed together, they form a homogeneous mixture. On the other hand, mercury and copper are both liquid, but when mixed, they form a heterogeneous mixture. Miscibility is another way to classify mixtures, depending on how well they can be mixed. Homogeneous mixtures have a uniform composition, such as sugar dissolved in water, while heterogeneous mixtures, like air, sand, and common salt, are not evenly mixed. To separate components of mixtures, we can use simple methods like hand-picking, sieving, and winnowing for heterogeneous mixtures, and more complex techniques like evaporation, centrifugation, decantation, sublimation, chromatography, and distillation for more complex mixtures. These methods have practical applications, such as extracting butter from curd or separating pigments from flower petals. Understanding these methods is important for effective separation of mixture components. This concludes our discussion on mixtures and in the next slide, we will focus on pure substances and their properties. Visit myCBSEguide.com for more material..
Scene 3 (3m 29s)
[Audio] Today we will be discussing the concepts of pure substances and mixtures, specifically focusing on two important processes: magnetic separation and concentration of solution. Starting with magnetic separation, this process utilizes a magnet to separate magnetic iron pins from non-magnetic sand. The second process, concentration of solution, measures the amount of solute in a given amount of solvent and can be expressed as either mass by mass percentage or mass by volume percentage. In simpler terms, this refers to the ratio of solute to solvent in a solution. Moving on, there are three types of solutions based on the size of solute particles: true solutions (particles less than 1 nm), colloidal solutions (particles between 1 nm and 1000 nm, visible under a microscope), and suspensions (larger particles that can be seen with the naked eye and cannot pass through a filter paper). Some examples of suspensions include muddy water and chalk in water. Colloidal solutions are heterogeneous mixtures with a dispersed phase (smaller proportion) and dispersion medium (larger proportion). These particles are large enough to scatter light, giving them a cloudy appearance. Some examples of colloids are milk, fog, and smoke in the air. In the next slide, we will further explore the characteristics of pure substances and mixtures. Thank you and onto the next slide..
Scene 4 (5m 8s)
[Audio] In this lesson, we will cover the defining characteristics of pure substances and mixtures and how they differ from each other. Slide number four will discuss the visible path of these concepts. Pure substances contain only one type of particle, while mixtures have a variety of different particles. The difference between them can be seen in their behavior when light passes through them. Pure substances have a clear path, while mixtures create an obstructed path due to the presence of colloidal particles that move randomly, known as Brownian movement. Mixtures can also be differentiated based on the amount of solute present, with unsaturated, saturated, and supersaturated solutions. They can also be classified based on the nature of the solvent, with aqueous and non-aqueous solutions. Next, we will discuss physical and chemical changes. Physical changes do not result in the production of a new substance, while chemical changes do. The lesson on pure substances and mixtures is now complete, and we hope it was informative. See you on the next slide..
Scene 5 (6m 16s)
[Audio] Today, we will discuss the defining characteristics of pure substances and mixtures, as well as methods of differentiation. Firstly, let's start with the definition of a pure substance. A pure substance is a material composed of two or more chemical elements, at least one of which is a metal, and cannot be separated into its components by physical methods. In contrast, mixtures are combinations of different substances that retain their individual properties, but may have varying compositions. One of the major benefits of mixtures is the creation of alloys, which combine different metals with varying characteristics to produce a stronger, more flexible, or desired end product. For instance, aluminum and copper alloys are used in automotive and electrical industries, respectively, while stainless steel and titanium alloys have various applications in commercial and aerospace settings. As students, understanding the different types of mixtures, methods of separation, types of solutions, and concentration terms is important, as it helps us appreciate the significance of alloys in different industries. It is also worth mentioning that the material presented in this speech was downloaded from myCBSEguide.com. We hope this presentation has provided you with a deeper understanding of pure substances and mixtures. Thank you for your attention, and we hope you have a better understanding of the topic. Have a good day!.